Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0052]
Towards a Selective Activation of Functional Groups Using Monochromatic Light.
Celine Helgen and Christian G. Bochet
Department of Organic Chemistry, University of Geneva, CH-1211 Geneva 4, Switzerland
E-mail: [email protected]
Received: 31
July 2000 / Uploaded: 1 August 2000
1. Introduction
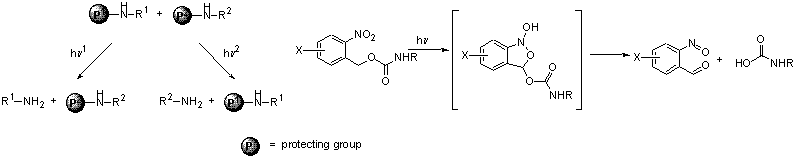
Selectivity has always been a major goal in any chemical processes, and particularly in organic synthesis. It is thus not surprising that more and more selective reagents have been developed in the last few decades. In photochemical reactions, the light can be considered as one of the reactants. Among the parameters characterizing the light, the wavelength is undoubtedly the most easily tunable one. This project aims at developing a set of functional groups that can be selectively activated by monochromatic light at various wavelengths, either to form bonds (eg. photoacylation) or to break bonds (eg. photodeprotection).
2. The goals
Preparation and evaluation of selective photolabile groups

We anticipate the substituents on the aromatic ring to have a strong influence on the position l of the absorption, its intensity e and the cleavage quantum yield F. By the proper tuning of these parameters, mutually orthogonal photolabile protecting groups should be available.
Optimisation of a clean and mild photoacylation reaction
Our next endeavour aims at developing a wavelength-selective acylation reaction by binding a photoactivable leaving group to a carbonyl. This function is expected to be stable towards nucleophiles under dark conditions, but to be highly reactive under irradiation at the proper wavelength. The parameters l, e and F will be adjusted to achieve selectivity.
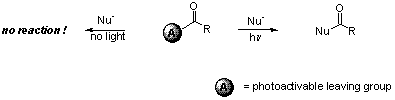
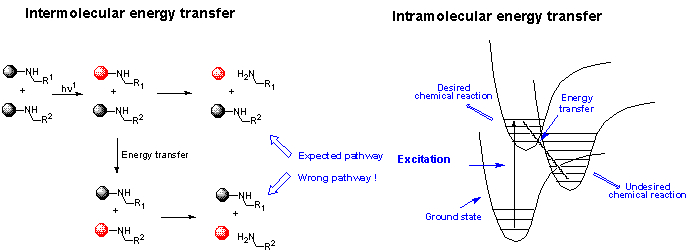
3. The challenges
The following requirements have to be fulfilled:
The energy transfer, either inter- or intramolecular, is of crucial importance. The initial excited state can indeed relax into a lower energy state. The information embedded into the original excitation energy is then lost. Relaxation can operate through several mechanisms, also at long distances and/or high dilution. In some cases, it can even totally inhibit the reaction.
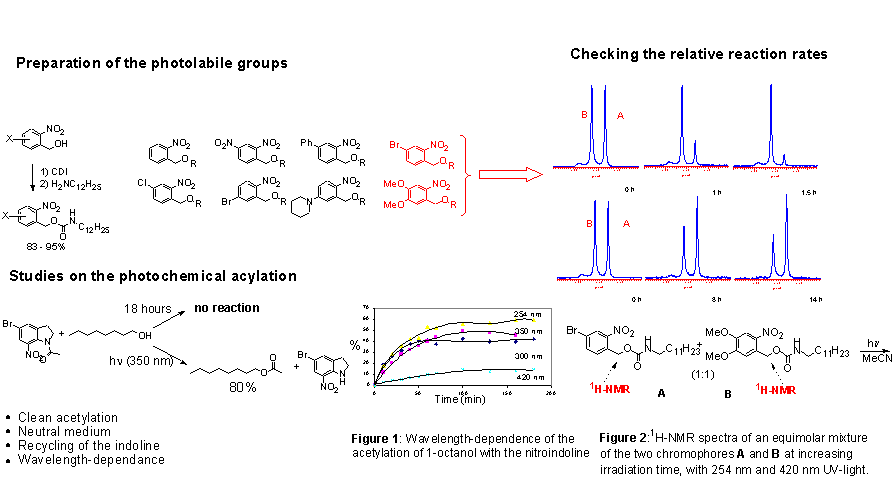
4. The results
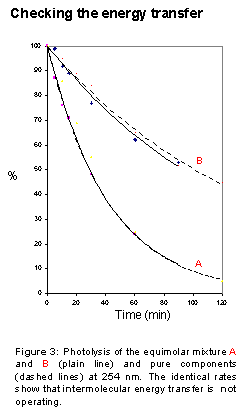
5. The Applications
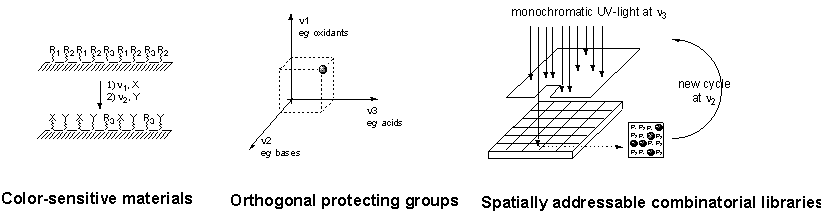

6. References
C.G. Bochet, Tetrahedron Lett. 2000, 41, 6341.
A. Patchornik, B. Amit, R.B. Woodward, J. Am. Chem. Soc. 1970, 92, 6333.
S. Pass, B. Amit, A. Patchornik, J. Am. Chem. Soc. 1981, 103, 7674.
P.J. Kocienski, Protecting Groups, Georg Thieme 1994.
S.P.A Fodor, J.L. Read, M.C. Pirrung, L. Stryer, A.T. Lu, D. Solas, Science, 1991,
251, 767.
N. Turro, Molecular Photochemistry, Benjamin Cummins 1978.
V.N.R. Pillai, Org. Photochem. 1987, 9, 225.
7. Acknowledgements
Pr. Alexandre Alexakis
Swiss National Science Foundation
Fonds Frederic Firmenich et Philippe Chuit
Janggen-Pohn Foundation
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0052 as the message subject of your e-mail.