Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0053]
Synergetic effect of urethane formation
Sergiu Coseri*, Adrian A. Caraculacu
Institute of macromolecular chemistry “P.Poni”, Gr. Ghica Voda Alley, 41 A, 6600, Iasi, Fax. 040-32-211299, Romania
E-mail: [email protected]
Received: 14 July 2000 / Uploaded: 29 July 2000
Abstract
The kinetic of reaction between isocyanates and alcohols is determined in a great extent by the presence in the reaction system of substances able to inhibit or to activating reaction rate of urethane formation.
The most important effect is produced by compounds that appear as reaction products (urethanes, urea).
By using a HPLC (High Pressure Liquid Chromatography) method to follow the evolution of reaction composition in usual condition of urethane synthesis, as like amine actions, by introduce certain amounts of amide or urea in the reaction system, this catalysed reaction rate of urethane formation.
The same effect the urethane addition present also, but in this case the urethane hydrogen atom presence is necessary.
Introduction
The problem of the hydrogen bonding role in the reaction of isocyanate with alcohols or glycol’s concern many authors.1-4
In this work, we proposed the following effect of introduction of different compounds type addition (urethanes, amides, amines, urea) on kinetic course, in the butanol (1-BuOH) - phenylisocyanate (PhNCO) reaction system.
The evolution of reaction rate was monitoring by using HPLC method, which allowed us to determine the concentration of all molecular species in the reaction.
The second order kinetic equation was checked, as described previously.5-7 Necessary model substances for the kinetic study by HPLC were synthesised according with literature data, Table 1.
Table 1. Model compounds.
No. |
Type: substitutes N-urethane R1-NH-COOC2H5 R1 |
Notation |
Reference |
1. |
C6H5 - |
CEE |
8 |
2. |
C2H5 - |
ECE |
9 |
N,N - urethane R1(R2)N-COOC2H5 R1 R2 |
|||
3. |
C6H5 - CH3- |
MCEE |
10 |
4. |
C2H5 - C2H5- |
ECEE |
11 |
N,N’ Disubstituted Urea R1-NH-CONH-R2 R1 R2 |
|||
5. |
C4H9- C4H9- |
DBU |
12 |
6. |
C6H5- C6H5- |
DFU |
13 |
Amides R1(R2)N-COH R1 R2 |
|||
7. |
C2H5- H- |
EFA |
14 |
8. |
C6H5- H- |
FFA |
15 |
Experimental
Materials. “Anala R” PhNCO pur. 99.9 % verified chromatographically was used. Analytical grade solvent, benzene, were dried on sodium wire and lithium hydride. All the reactants and solvent were freshly redistilled immediately before the solutions were made.
Kinetics. A similar technique to that described previously for PhNCO - alcohols was used.1
HPLC analysis. A Hewlett-Packard 1084 A chromatograph fitted with UV variable wavelength detector 1030 B was used.. The reverse phase method was preferred using Lichrosorb RP-18 and respective RP-8 columns 10 m m (Brownie-Labs, USA) of 250 mm length and 4.6 mm internal diameter. Injection volume 5 mm3, column temperature 400C, flow 1 cm3 min-1.
In some cases, for improved HPLC separation, the benzene (Bz) from the solvent mixture (Bz-methanol) was removed by evaporation prior to analysis, thus reducing the volume of the solution by 75 %. The initial concentration was restored with pure methanol (MeOH). Total removal of the solvent is not recommended, to avoid the risk of losing some of the methylurethane by partial sublimation.
Results and discussion
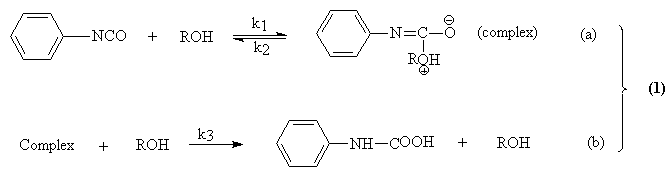
Baker and co-workers16-18 was the first who extensively studied the kinetic of reaction between alcohols and monoisocyanates. Authors used the second order kinetic treatment, despite of the unconcordance between the requirements of the second order mechanism and the observed reaction rate constant dependence on the initial reactants concentration. Baker observed also an autocatalytic effect produced by the formed urethane, according with the follow mechanism of reaction:

The autocatalytic effect of the urethane formation was to question long time. In the first part of this paper we definite obviously that the urethane apparition in the reaction mixture, produced an enhanced of reaction rate.
By studying the kinetic of reaction between PhNCO and ethanol (EtOH), with large excess of EtOH, we can create the conditions of pseudofirst order of reaction.
We effectuate parallel, other kinetic study of the same reaction. In this case, the increased concentration of alcohol out of first case, was substitute through addition in the system by carbanilic acid ethyl ester (CEE), in fact the product of this reaction.
As we shown from Table 2, that the addition of CEE act similar with the increased of initial concentration of EtOH.
Table 2. Influence of the urethane addition by rate constant k, between EtOH and PhNCO.
Nr. crt. |
Isocyanate concentration (CPhNCO) |
Alcohol concentration (CEtOH) |
Urethane (ECE) addition concentration (CCEE) |
S ( CEtOH + CCEE) |
k * 103 mol-1 min-1 dm3 |
1. |
0,0058 |
0,10 |
0 |
0,10 |
4,4 |
2. |
0,0058 |
0,20 |
0 |
0,20 |
8,6 |
3. |
0,0047 |
0,13 |
0,10 |
0,23 |
18,4 |
4. |
0,0058 |
0,53 |
0 |
0,53 |
40,6 |
5. |
0,0047 |
0,14 |
0,39 |
0,53 |
32,69 |
6. |
0,0058 |
0,88 |
0 |
0,88 |
56,98 |
7. |
0,0047 |
0,13 |
0,80 |
0,93 |
63,18 |
8. |
0,0047 |
0,13 |
1,7 |
1,83 |
102,3 |
The addition of another aliphatic urethane, such as carbanilic acid N-ethyl urethane (ECE), produced an enhanced much more (abb. four times) of reaction rate, toward the case in when the aromatic urethane (CEE) was addition, Table 3, nos 2,3.
Table 3. Reaction of PhNCO with 1-BuOH at 600C, in benzene, k x 103 (mol-1 min-1 dm3 ).Nr. crt.Concentra]ion of the addition species, (mol dm-3) |
k x 103 |
|||||||
CPhNCO |
C1-BuOH |
|||||||
1. |
0.1758 |
0.3502 |
- |
- |
32.92 |
|||
2. |
0.1739 |
0.3506 |
ECE |
0.6867 |
311.88 |
|||
3. |
0.1792 |
0.3506 |
CEE |
0.7092 |
78.32 |
|||
4. |
0.1761 |
0.3491 |
MCEE |
0.7029 |
16.16 |
|||
5. |
0.1739 |
0.3550 |
ECEE |
0.6956 |
36.23 |
|||
6. |
0.1739 |
0.3625 |
FFA |
0.7092 |
252.03 |
|||
7. |
0.1747 |
0.3501 |
EFA |
0.7093 |
584 |
|||
8. |
0.1706 |
0.3496 |
DMF |
0.7034 |
105.36 |
|||
9. |
0.1634 |
0.3564 |
DFU |
0.0032* |
48.49 |
|||
10. |
0.1760 |
0.3684 |
DBU |
0.7084 |
1222 |
|||
11. |
0.1760 |
0.3424 |
TEA |
0.7011 |
4014 |
|||
*We work with small concentration of DFU, due to the low solubility in these conditions; TEA = (C2H5)3N;
Provided, in the reaction system of substituted urethane is introduced, we showed (Table 3, nos. 3,4) that the reaction rate value tends to diminish.
This behaviour can be explained by hydrogen since N atom absence, and this H atom is able to give associates by hydrogen bond with the alcohol, therefore activating the reaction.
The insertion in the analysis reaction system of amides, considerably enhanced reaction rate, as can be seen from Table 3, nos.6,7. The most powerful effect is present by the aliphatic amides follow than aromatic amides.
Surprisingly, N,N-substituted amides manifest a pronounced catalytic effect, to difference than substituted urethanes.
This situation can be explained by supposing that in the dimethylformamide (DMF) case, two opposite effects take place. In the first, DMF inactivates the alcohol reactivity due to the appearance of hydrogen bonding between an OH group and DMF, equation 2.

The second effect of DMF is that of activation of the PhNCO by -NCO group polarisation presented by structures a in equation 3. At higher temperatures the interaction between -NCO and DMF can ease even total movements of electrons, resulting in the formamidinic group appearance, (eq. 3).
In our kinetic conditions, the formamidine is not observed, the precision of our detection could even determine 0,32 x 10-2 mol / l formamidine.
Regarding these two effects, the experimental results presented above demonstrate that during the reaction between PhNCO and Bu OH, the activating effect of DMF is prevalent.
Along the same time, another stage of this work was that varied ureas was added in the PhNCO - BuOH reaction system. The very important think is to know how is the urea impact on the isocyanate - alcohol reaction rate; possible ureas traces from reaction mixture can considerably influenced the reaction rate. Indeed, the addition of very little quantities (almost 220 times little toward previous addition) since the solubility limit of DFU in Bz, lead to an enhancement of the rate constant with 15%, Table 3, no. 9.
The activation of urethane reaction is significant as well as aliphatic urea addition, like dibuthyl urea (DBU), Table 3, no. 10.
The most growth of reaction rate value of all addition used, was observed in the case of triethylamine, Table 3, no. 11.
References
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0053 as the message subject of your e-mail.