Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0055]
Synthesis of Isoguanosine Analogues
Marjan Jeselnik1, Kobe Joze2
1
Faculty of Chemistry and Chemical Technology, Askerceva 5, SI-1000 Ljubljana2
National Institute of Chemistry, Hajdrihova 19, SI-1000 LjubljanaReceived: 1
August 2000 / Uploaded: 2 August 2000
INTRODUCTION
An increasing number of isomeric (unnatural) nucleosides and nucleotides bearing an isomeric backbone, sugar or base have been characterized.1
A nucleobase isomer like isoguanine apparently makes feasible a non-standard base pair between 2`- deoxyisocytidine and 2`-deoxyisoguanosine when incorporated into oligomers. Some levels of functions by that isomeric replacement are retained and in some instances new functions are formed.2 The results reported by Switzer et al have shown that various polimerases handle this nonstandard base pair while Horn et al3 reported on the pairing in the absence of enzyme. Herein we report on a subtle change in a molecular shape by replacing N3-nitrogen with more lipophylic CH3-group and C8-H by N8-nitrogen.Though we do not prevent the Watson-Crick pairing the ring stacking might be altered. The absence of N3-nitrogen introduces an incorrectness that would disrupt the minor groove spin of hydration what means that should eliminate the presence of any ordered water molecules or metal ions at this sequence position.4 Recently,5 it has been demonstrated that in dA-dT sequences, the spine of hydration should be considered an integral part of the helix and the possible metal ions binding in the minor groove is critical for the structural integrity of duplex DNA.
In the light of these statements, our intention to design and carry on efficient synthesis of 3-deaza-8-azaisoguanosin (2), seemed attractive and significant especialy when additional self-assembled properties are considered. G-quartets covalent assemblies6 selectively coordinate monovalent and divalent cations to give extremely stable complexes, while the lack of one potential H-bond may considerably alter the binding affinity and selectivity of this potential ionophores.

RESULTS AND DISCUSION
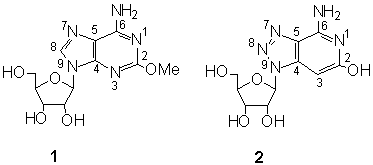
In spite of remarquable interest in isoguanosine and the 2-O-methylated congener spongosine7 (1), there have been no attempts to the synthesis of 3-deaza analogues. Our efficient8 high yield procedure for the preparation of 3-deaza-8-aza-guanosine analogues enabled us to select one of the two possible routes. The first would follow the obvious transformation of 11 , while the second via the intermediate 3 offered better option since 3 is readily transformed to compounds 5 or 6 (Scheme 1).
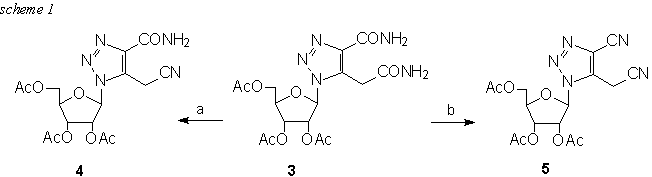
Reagents: (a) 1.1 eq (CF3CO)O, pyridin, dry THF, -10-0�C ; (b) 2.2 eq (CF3CO)O, pyridin, dry THF, -10-0�C
Intramolecular cyclisation between a cyanomethyl group and an adjacent cyano group located on imidazole9 ring proceeded well by acid-catalysis with anhydrous hydrogen bromide in one direction when the aromatic nitrile becomes the ring nitrogen. This condensation has been reported as successful on a free base only, whether the alkoxide condensation of a dinitrile as was reported for the synthesis of aminoalkoxynaphthyridines10 furnished the desired product just using sodium methoxide. This condensation employed for our purpuses is presented in Scheme 2.
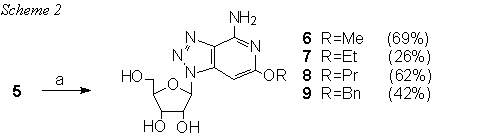
Reagents: NaOR in ROH (R=Me, Et, Pr and Bn)
This is the first successful example in nucleoside chemistry presenting a novel and efficient procedure in the chemistry of purine analogues mimicking guanosine framework. The use of different sodium alkoxides afforded 2-alkoxy-3-deaza-8-azaadenosine series 6-9 prepared as potentially coronary vasodilatatoris. Besides intermediate 9 is the anticipated precursor to provide the target nucleoside 2. (Scheme 3).

Reagents: (a) H2 / 5% Pd/C, 80% EtOH
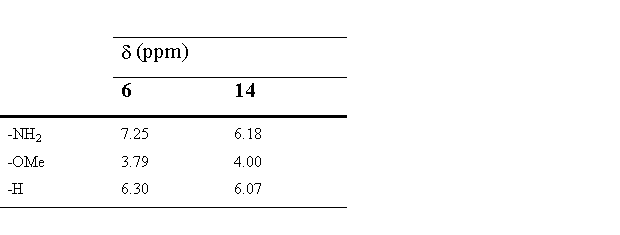
Comformation of obtained products was unambigously determined by the comparison of 1H spectral data of 6 and 14. (Table 1)
Table 1.
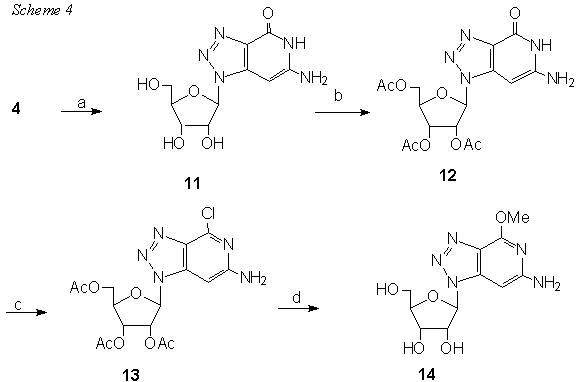
Reagents: (a)1M NEt3 in 80% MeOH; (b) Ac2O, pyridin, DMF; (c) POCl3, collidine, NEt4Cl, MeCN; (d) MeONa in MeOH
The intermediate 13 is an interesting precursor. After careful hydrogenation it affords 15 and 4,6-diamino-9(b -D-ribofuranosil)-1,2,3-triazolo[4,5-c] pyridine 16 was obtained in liquid ammonia at 110� (Scheme 5)

Reagents: (a) H2 / 5% Pd/C; (b) NH3 (l), 110�C
CONCLUSIONS
A novel alkoxide condensation of the new dinitrile synthon 2-[ 1-(2,3,5-tri-O-acetyl-b -D-ribofuranozil)-4-cyano-1,2,3-triazolo-5-il] acetonitrile was successfully applied to the synthesis of 3-deaza-8-aza isoguanosine analogue 4-amino-1-(b -D-ribofuranozil) triazolo[ 4,5-c] piridin-6(5H)-on. New spongosine analogues are reported as well.
ACKNOWLEDGMENTS
This investigation was supported by the Ministry of Sciece and Technology of Slovenia (Grant No. 33-030 and CI-01513) and the EU (BIOMED1, Agreement No. ERBCIPDCT 930194, PL 93-1112). We thank dr. Bogdan Kralj at the Mass Spectrometry Centre at Jozef Stefan Institute (Ljubljana) and dr. Janez Plavec at the Slovenian National NMR centre.
REFERENCES
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0055 as the message subject of your e-mail.