|
Fourth International Electronic Conference on
Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0066] |
|
|
|
1. General Aspects
|
|
Azidohetarenes have found large interest because of its reactive
azido group which gives a
number of reactions which can be used synthetically [1]. We have prepared previously
a number of hetaryl azides [2] having reactive substituents such as
phenyl-, acyl-, hydrazono- and nitro groups
in ortho-position which were used as synthons
for thermolytic induced heteroelectrocyclic ring closure reactions.
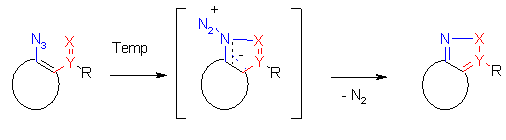
In this contribution we studied the thermal behavior of azidoarenes and azidohetarenes with and without reactive ortho-substituents to get insights in the thermal properties of these compounds which are interesting and important for thermal reactions.
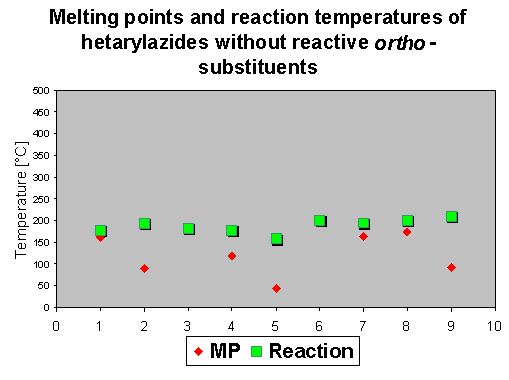
By means of differential scanning calorimetry (DSC) we obtained besides melting points (which are in many cases of interest because the purity can be better determined than with chromatographic methods) the temperature areas for reaction or decomposition [3]. This information is very valuable because it allows the synthetic chemist to plan the reaction conditions (e.g. to select suitable solvents and reaction temperatures which are high enough for a quick reaction, but below subsequent decomposition or rearrangement temperatures). Integration of the reaction and decomposition peak area gives information about the reaction enthalpy which in turn is a very important safety information. Together with the temperature it shows if and at which temperature a particular substance is an explosive, which is in azide chemistry of great value.
|
|
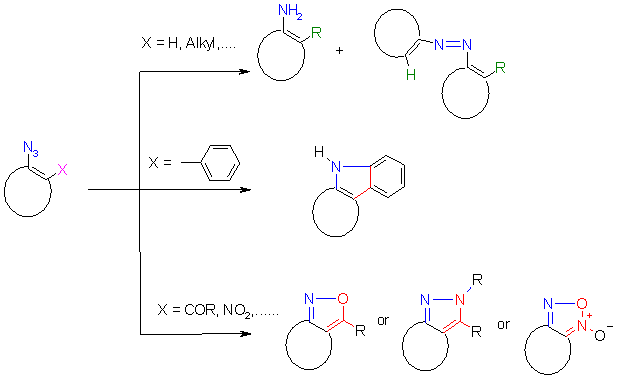
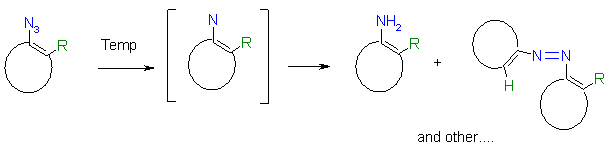
2. Decomposition of azidohetarenes without reactive ortho-substituents to nitrenes
|
|
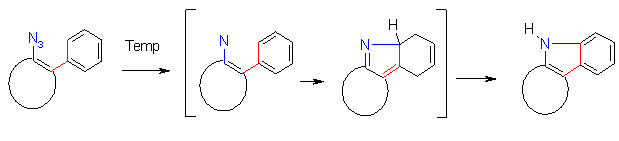
Azides are known to give on thermolysis singlet nitrenes (or by intersystem crossing triplet nitrenes) which react with hydrocarbon compounds to form e.g. a C-N bonding by C-H- insertion, or a N-N bonding by reaction with nucleophiles to give ring enlargements or amines. [1]. By dimerization e.g. the formation of colored azo compounds is observed.
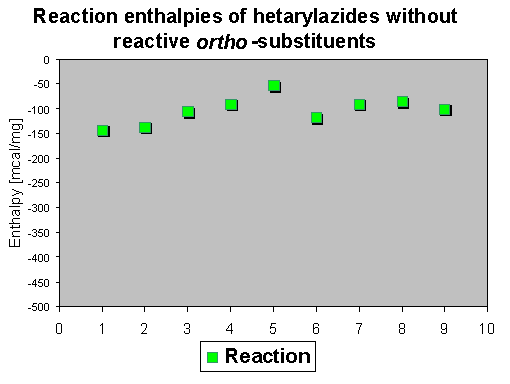
The values for the reaction enthalpy are in an average range between - 50 and - 150 mcal/mg, which means that handling of these compounds bears no special danger.
|
|
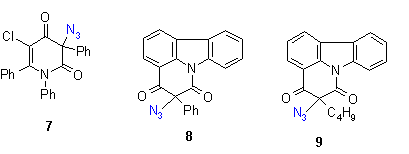
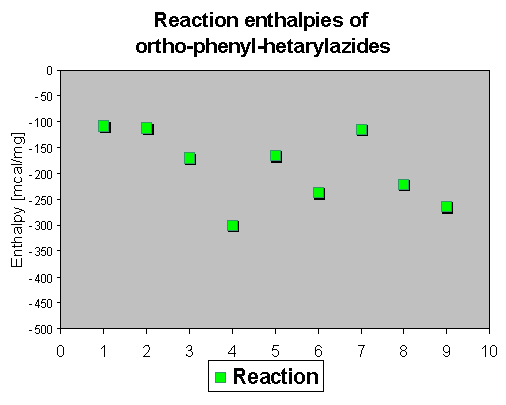
3. Decomposition of azidohetarenes with ortho-phenyl substituents to indoles
|
|
Thermolysis of azidohetarenes with ortho-phenyl groups is described to lead in the first step to
hetaryl-nitrenes which cyclize by an electrocyclic reaction followed by a 1,5-hydrogen shift to give indolo-fused heterocycles
[1]. This reaction sequence offers a simple way with high yields
to this interesting class of compounds.
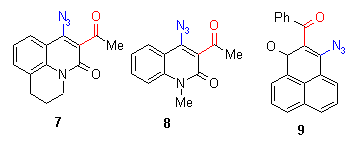
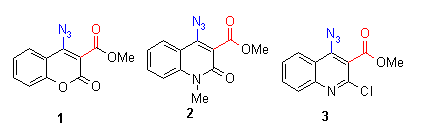
The azido compounds were obtained by the procedures described in earlier reports [2]. We could show that also azides with benzyl substituents such as compound 1 give in moderate yields the corresponding quinoline compounds. The phenyl compounds (2-9) react in good yields to indolofused heterocycles[2].
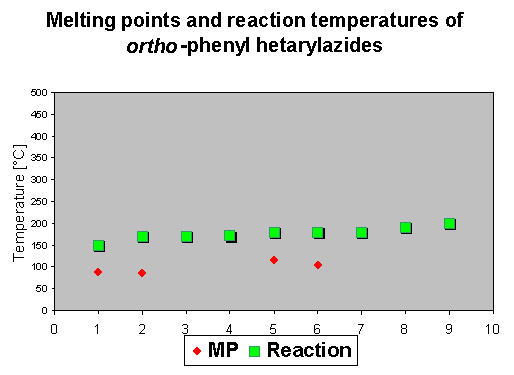
As the first step - similar to the compounds described in chapter 2 - a nitrene is described to be formed by thermolysis, which means that there should be no influence on the decomposition temperature. It was of interest to check if there is an influence visible of the ortho-phenyl group to the the reaction enthalpies. Because of its reactivity, only a few compounds show a mp. The reaction temperature ranges again between 150 and 200 oC, which means that actually the ortho-phenyl substituent does not change the initial reaction temperature.
The reaction enthalpies of the azides 1-9 have rather different values between - 110 and - 300 mcal/mg. So this assay does not allow a general statement on further activation influence of the phenyl group, because substituent effects and the heterocyclic system seem to superimpose the effects of the phenyl group.
The rather high enthalpy values beyond - 200 mcal/mg in some of the compounds give hints that this class of compounds must be carefully handled - especially if large scales are thermolyzed. |
|
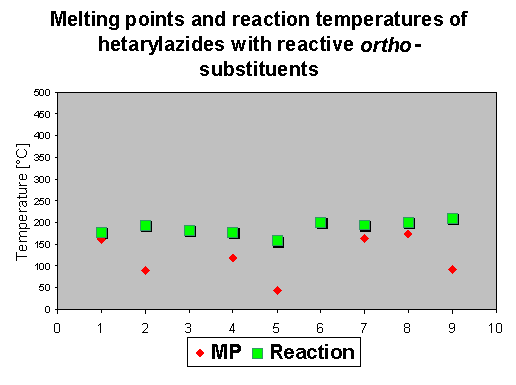
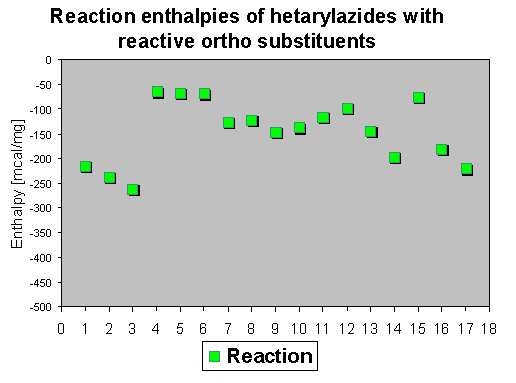
4. Decomposition of azidohetarenes with reactive ortho-substituents to isoxazoles, pyrazoles and furoxanes
|
|
Ring closure reactions of an azido group with ortho-acyl groups has been described to proceed
not via a nitrene mechanism [1], but a
heteroelectrocyclic (or pseudopericyclic) mechanism is
postulated [4] involving as the first step a cyclization followed in a second step by loss of
nitrogen. As reaction products isoxazoles, pyrazoles or furoxanes are obtained, which offers a simple approach to this
interesting class of compounds. This reported reaction sequence means that - in contrast to the 2
preceeding chapters - the first reaction step (and also the reaction temperature) involves the
attack of the azide as an electrophile at the negative carbonyl oxygen of the acyl group.
It was of interest whether this mechanism is visible in the reaction temperature by lower temperatures, or if the first activation step needs a similar activation energy as been found in the preceeding chapters.
The compounds were obtained by the procedures described in earlier reports [2].
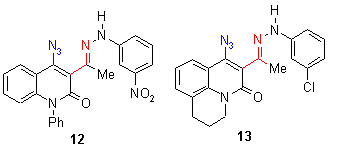
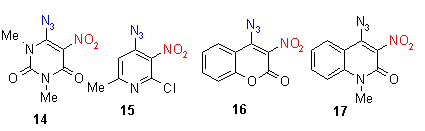
None of the investigated aldehydes (4-6) and ketones (7-9) show a mp, because the reactive substituent causes a quick reaction at rather low temperatures of 120 - 160 oC, which means that in this case the reaction temperature was lowered for about 30oC. Analogous esters (1-3) have all a mp, the reaction temperatures are similar to the preceeding compounds in chapter 2 and 3 between 150-180 oC. Azidohetarenes with ortho-hydrazono substituents (10-13) have no mp and cyclize between 130-180 oC by thermolysis to give a five-membered pyrazolo ring (and not a sixmembered triazino ring) [2]. Ortho-nitroazides (14-17) have again no mp and give between 110 and 190 oC the fivemembered furoxanes (oxadiazolo-3-oxides) [2].
The reaction enthalpies of the acyl compounds 4-9 are between - 70 and - 150 mcal/mg, the reaction enthalpies of the esters 1-3 range between - 50 and - 240 mcal/mg. It can be found that methyl esters have high enthalpy values (between -200 and - 260), whereas ethyl esters (not shown in the diagram) range between -50 and - 160 mcal/mg. The enthalpy values of the hydrazono compounds 10-13 range between 100-140 oC. The enthalpy values of ortho-nitroazides (14-17) are between - 50 and - 200 mcal/mg. So this assay does again not allow a general statement on further activation influence of the reactive ortho group, because different group and substituent effects seem to superimpose the effects of the reactive ortho group. The rather high enthalpy values - beyond - 200 mcal/mg - of some compounds give hints that this class of compounds must be handled carefully - especially if such azides are thermolyzed in large scales.
|
|
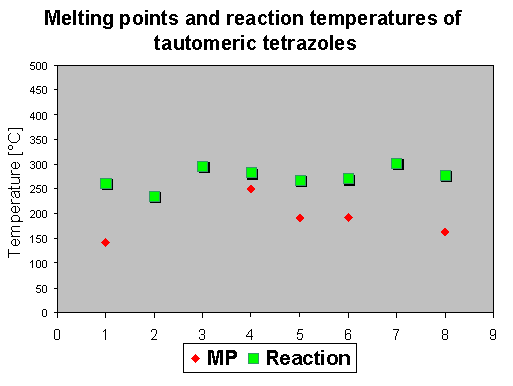
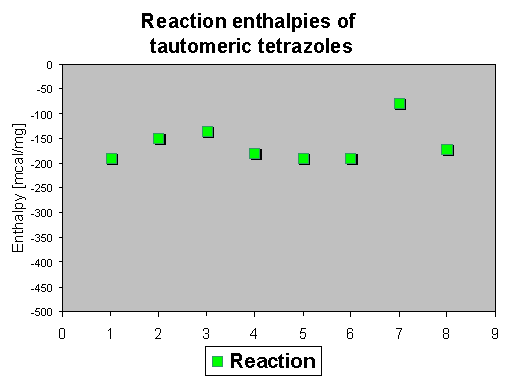
5. Azido-tetrazolo tautomers
|
Heterocyclic azides in the ortho-position of ring nitrogens are known to exist in a high percentage as the corresponding tetrazolo tautomers
[5, 2e, 2f, 2m]. In this assay the thermal behavior of azides was investigated, which are known to exist mainly in the tetrazolo form. It was of interest if these tetrazoles could react at higher temperatures in its azido form and if the reaction or decomposition temperatures are in the same region as found for azidohetarenes.

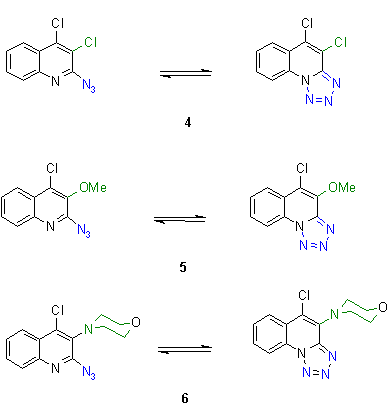
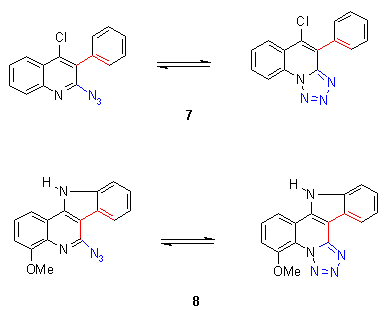
The reaction temperatures of tetrazolo-hetarenes were generally found at significantly higher values, between 220-330 oC. Preparative investigations revealed that no defined reaction products were found, e.g. the corresponding indoles from 7 and 8 [6].
The reaction enthalpies are in the average range between - 70 and - 180 mcal/mg, but synthetic assays [6] reveal only decomposition reactions, which could not used for preparative purposes.
The findings in the tetrazolo field show that tetrazoles which do not show any azido moiety cannot be used as masked azides. There could not be found any useable synthetic reaction, a result which is also supported by the findings when we tried to reduce the tetrazolo moiety to amines in analogy to azides. In all cases the tetrazolo moiety remained unchanged [2] except when a Staudinger reaction [5]was used cleaving the tetrazole with triphenylphosphane. |
|
6. Conclusion
|
|
The DSC diagrams show that the decomposition of hetarylazides occurs generally between 80
and 200 oC, and the influence of an reactive ortho group is rather small - it does not exceed more than 30 oC.
The enthalpies which range between - 50 and - 300 mcal/mg show that reactive ortho groups increase in some cases (depending on further substituent influences) the values which should be interpreted as a safety hint, because on scaling up a reaction this can produce explosions. It is not possible to obtain reliable data for general mechanistic statements because there are substituent effects which superimpose the different reaction enthalpies. Exact statements are restricted exactly to one particular system. |
|
7. Experimental
|
|
General preparation of the azides used in this work:
The preparations isare described in detail in ref. [2]. A standard procedure starts from either the corresponding chloro- or tosyloxy compounds, which were reacted with sodium azide in dimethylformamide or N-methylpyrrolidone at temperatures between 0 - 80 oC. After the reaction, the reaction mixture was poured onto ice, and the solid which precipitated was collected by filtration.
Measurements:
|
|
8. Acknowledgement
|
| This work was supported by the FWF (Österreichischer Fonds zur Förderung der wissenschaftlichen Forschung) project No. P 10785-CHE . |
|
9. References
|
|
[1] Azides and Nitrenes, Scriven E.F.V. (ed), Academic Press, Orlando, Florida, 1984; Scriven E.
F. V., Turnbull K., Chem. Rev., 88 (1988), 297; Iddo B., Meth-Cohn O., Scriven E. F. V., Suschitzky H., Gallagher P. T., Angew. Chem., 91 (1979) 965, Int. Ed. Engl., 12 (1979), 900.
[2] Stadlbauer W., Monatsh. Chem. 117 (1986) 1305-1323;
[3] Kappe Th., Stadlbauer W., Molecules 1 (1996) 255-263 [4] Bakulev, V. A. , Kappe, C. O. , Padwa, A. , Organic Synthesis: Theory and Applications, 3 (1996) 149 . [5] Sasaki, T., Kanematsu, K., Murata, M., Tetrahedron 28 (1972) 2383. [6] Gerhard Hojas, diploma thesis, University of Graz, 1997. |
|
All comments on this poster should be sent by e-mail to (mailto:[email protected]) [email protected] with A0066 as the message subject of your e-mail. |