|
 |
|---|
[A0069]
|
 |
|---|
Julio A. Seijas*, M. Pilar Vázquez-Tato* , Luis Barreiro-Castro
Departamento de Química Orgánica. Facultad de Ciencias de Lugo. Universidad de Santiago de Compostela. Aptdo.
280. 27080-LUGO.
SPAIN
E-mail: [email protected] , [email protected]
Received: 4 August 2000 / Uploaded: 5 August 2000
Our group has been investigating on the transformation
of styrene derivatives into ß-phenylethylamines [1]. We had
previously worked on the carbolithiation of styrenic compounds and we achieved
successfully the hydroxylation of the ![]() -position
of styrene [2]. However this method proved to be unsuccessful
when applied to addition of lithium amides [3].
-position
of styrene [2]. However this method proved to be unsuccessful
when applied to addition of lithium amides [3].
ß-hydroxy-ß-phenylethylamines are very interesting due to their wide
variety of pharmacological properties [4]. To achieve their
synthesis we had to change our strategy. Instead of leaving the introduction of the oxygen
atom at C-![]() for the end of the synthesis, we
decided to start from
for the end of the synthesis, we
decided to start from ![]() -hydroxystyrene
derivatives, where the oxygen atom was present previously to the nucleophillic addition
stage. Previously it had been reported that enolcarbamates could support the addition of
organolithium without reaction of the carbonyl group [5]. Thus
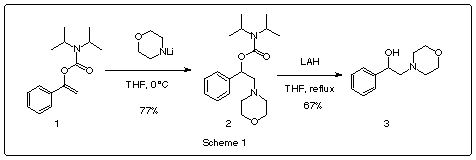
we prepared the N,N-diisopropylcarbamate of the enol of acetophenone 1. We
treated it with the lithium amide of morpholine obtaining the addition compound 2
in a 77% yield, the cleavage of isopropylcarbamates is performed by reduction with lithium
aluminum hydride [6], thus we obtained
ß-hydroxyl-ß-phenylethylamine 3 in 67% yield.
-hydroxystyrene
derivatives, where the oxygen atom was present previously to the nucleophillic addition
stage. Previously it had been reported that enolcarbamates could support the addition of
organolithium without reaction of the carbonyl group [5]. Thus
we prepared the N,N-diisopropylcarbamate of the enol of acetophenone 1. We
treated it with the lithium amide of morpholine obtaining the addition compound 2
in a 77% yield, the cleavage of isopropylcarbamates is performed by reduction with lithium
aluminum hydride [6], thus we obtained
ß-hydroxyl-ß-phenylethylamine 3 in 67% yield.
 |
Another interesting analogs of ß-phenylethylamines are
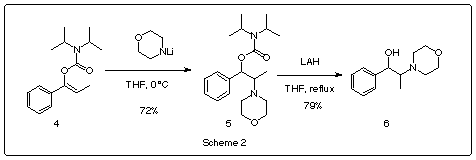
ephedrine like compounds. To carry out the synthesis of this skeleton, we prepared the
N,N-diisopropylcarbamate of the enol of propiophenone 4, this was reacted with the
lithium amide of morpholine affording addition product 5 in 72% yield, the cleavage
of carbamate group presenting 5 with lithium aluminum hydride gave amino
alcohol 6 in 79% yield.
 |
We think our method for the synthesis of ß-phenylethylamine
derivatives, constitutes a interesting approach with good yields. Actually we are checking
the scope of this methodology for a wide variety of primary and secondary amines.
Acknowledgments: Financial support from DGES (project PB96-0932) is gratefully acknowledged.
References:
1.- Seijas, J.A., Vázquez-Tato, M.P.; Entenza, C.; Martínez, M.M.; Ónega, M. G.; Veiga,
S., Tetrahedron Lett., 1998, 39, 5073-5076. ECSOC-3, 1999, [a0022]
"Microwave Enhanced Hydroamination of Styrene", Julio A. Seijas, M. Pilar
Vázquez Tato and M.Montserrat Martínez. ECSOC-3, 1999, communication [a0025]:
"Hydroamination of Cinnamyl Alcohol", Seijas, J. A.; Vázquez-Tato, M. P.;
Barreiro-Castro, L and Romero-Van-der-Schoot, R.
2.- Martínez, M.M.; Ónega, M.G.; Tellado, M.F.; Seijas, J.A.; Vázquez-Tato, M.P., Tetrahedron, 1997, 53, 14127-14130.
3.- M. Montserrat Martínez, Ph. D. Thesis, unpublished results
4.- "The Merck Index", 12th. edition. Merck and Co., Inc. Whitehouse Station, 1996.
5.- Hoppe, D.; Hense, T., Angew. Chem. Int. Ed. Engl., 1997, 36, 282-2316.
6.- Guarnieri, W.; Sendzik, M., Fröhlich, R.; Hoppe, D., Synthesis, 1998,
1274-1297.
 |
All comments on this poster should be sent by e-mail to (mailto:[email protected]) [email protected] with A0069 as the message subject of your e-mail. |  |