SYNTHESIS OF STYRYLAMINES FROM ß-METHOXYSTYRENE |
 |
[A0070]
Julio A. Seijas*, M. Pilar Vázquez-Tato* , Luis Barreiro-Castro and
M. Gabriela Ónega
Departamento de Química Orgánica. Facultad de Ciencias de Lugo. Universidad de Santiago de Compostela. Aptdo.
280. 27080-LUGO.
SPAIN
E-mail: [email protected] , [email protected]
Received: 4 August 2000 / Uploaded: 5 August 2000
In the last years we have been searching on the reactivity of
styrenic compounds towards nucleophiles [1], in this
communication we want to report he reactivity of beta-methoxystyrene towards butillithium
and lithium amides.
The addition of alkyllithiums and lithium amides to styrene usually is followed by the
addition of an electrophilic reagent that quenches the benzylic anion. (escheme 1).
 |
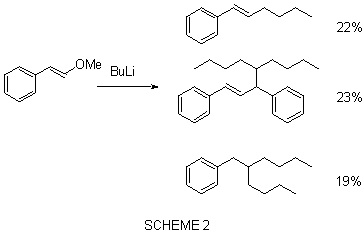
We found that addition of butyllithium to ß-methoxy
styrene (0şC/THF) it gave a mixture of products all of them coming from an
addition-elimination mechanism.(scheme 2). We tried to control the reaction to stop
it at the stage of formation of ß-butylstyrene but we did not have success.
 |
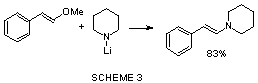
We checked also the reactivity with the lithium amide
of piperidine at 0şC in THF, but no reaction product could be isolated, we carried
out the reaction in dry ethyl ether and in this case, the addition-elimination was clean
and led to the corresponding enamine in a 83% yield. However when we treated the
same methoxystyrene with the lithium amide of butylamine, we did not observed
addition product.
 |
Actually we are trying to know the scope of this reaction. We think
this reaction would constitute a good entry to the preparation of styrylamines, these are
alkaloids present in some bromeliaceae, amaranthaceae, liliaceae, rutaceae and
sponge Axinyssa aplysinoides [2].
Acknowledgements: Financial support from DGES (project PB96-0932) is gratefully acknowledged.
References:
1.- Estévez, J.C.; Villaverde, M.C.; Estévez, R.J.; Seijas, J.A.; Castedo, L., Synthetic Commun, 1990, 20, 503-507. Seijas, J. A.; Vázquez-Tato, M.P.; Castedo, L.; Estévez, R.J.; Ruíz, M., J. Org. Chem., 1992, 57, 5282-5283. Martínez, M.M.; Ónega, M.G.; Tellado, M.F.; Seijas, J.A.; Vázquez-Tato, M.P., Tetrahedron, 1997, 53, 14127-14130. Seijas, J.A., Vázquez-Tato, M.P.; Entenza, C.; Martínez, M.M.; Ónega, M. G.; Veiga, S., Tetrahedron Lett., 1998, 39, 5073-5076.
2.-Blackman, A.J., Green, R. D., Aust. J. Chem.,1987, 40,
1655-1662. Blackman, A.J., Eldershaw, T.P.D., Garland, S.M., Aust. J. Chem.,1993, 46,
401-405. Compagnone, R.S.; Faulkner, J.D., J. Nat. Prod.,1995, 58, 145-148.
Greger, H., Zechner, G.; Hofer, O.; Hadacek, F.; Wurz, G., Phytochemistry,1993, 34,
175-180. Hinterberger, S.; Hofer. O.; Greger, H., Tetrahedron, 1994, 50,
6279-6286. Prakash, D.; Raj, K.; Kapil, R. S.; Popli, S. P., Indian J. Chem., Sect. B,1980,
19, 1075-1076. Lin, J.-H., Phytochemistry,1989, 28, 621-622. Milner, P.H.;
Coates, N.J.; Gilpin, M.L.; Spear, S. R.; Eggleston, D.J., J. Nat. Prod.,1996, 59,
400-402. Greger, H.; Zechner, G.; Hofer, O.; Vajrodaya, S., J. Nat. Prod.,1996, 59,
1163-1168.
 |
All comments on this poster should be sent by e-mail to (mailto:[email protected]) [email protected] with A0070 as the message subject of your e-mail. |  |