Fourth International
Electronic Conference on Synthetic Organic Chemistry (ECSOC-4),
www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0073]
A New Approach to the Synthesis of Hydrogenated Pyrimidine-2-imines
Department of Organic Chemistry, State Academy of Fine Chemical Technology,
Vernadsky Avenue 86, Moscow 117571, Russia
Phone/Fax (095) 431-6332, E-mail: [email protected]
Received: 7 August 2000 /Uploaded: 12 August
Abstract: A new convenient method for the synthesis of hydrogenated
2-cyaniminopyrimidines has been developed. This method is based on preparation
of a-tosyl substituted N-cyanoguanidines 11
followed by reaction with enolates of a-functionally
substituted ketones to give 5-functionalized 2-cyanimino-4-hydroxypyrimidines
12, 13, 15, 16. All the obtained hydroxypyrimidines
are readily converted into the corresponding 5-functionalized 2-cyanimino-1,2,3,4-tetrahydrohydropyrimidines
17-20 by heating in the presence of acids. Treatment of 5-acetyl-4-hydroxypyrimidines
12 with aq. KOH gives 4-hydroxypyrimidines 21 in result of
removing the acetyl group.
Keywords: N-cyanoguanidine, N-cyano-N'-(1-tosyl-1-alkyl)guanidines,
a-functionally substituted ketones, 2-cyanimino-4-hydroxyhexahydropyrimidines,
2-cyanimino-1,2,3,4-tetrahydropyrimidines.
 Introduction
Introduction
 Results
and Discussion
Results
and Discussion
 Conclusion
Conclusion
 References
References
 Introduction
Introduction
Last years a large variety of compounds bearing a guanidine function with
very interesting biological activities was isolated from natural products.
The isolation and structure determination, synthesis, biosynthesis and
the biological properties of such compounds were the subject of numerous
reports (for reviews see [1-4]). Considerable attention has focused on
natural and synthetic heterocycles containing a guanidine group. Many of
these heterocyclic alkaloids were isolated from marine organisms. Typical
representatives of these alkaloids are tetrodotoxin 1, ptilocaulin
2, saxitoxin 3, batzelladine B 4, crambescin B 5
and many others. All these alkaloids have a hydrogenated pyrimidine ring
with 2-imino (or 2-amino) group.

The abundance of heterocyclic guanidine natural alkaloids which exhibit
a broad range of biological properties has stimulated the development of
various methods for their synthesis as well as synthesis of their analogs
[1-4]. However, these methods suffer from their low universality. Really,
they give possibility to synthesize only discrete compounds, and no series
of related compounds for biological testings. Thus, the need for the development
of new and general methods for heterocyclic guanidines synthesis, particularly
hydrogenated pyrimidine-2-imines is of considerable importance.
Recently we have developed a convenient general method for the synthesis
of 5-functionally substituted 4-hydroxyhexahydro- 8 and 1,2,3,4-tetrahydropyrimidine-2-thiones/ones
9 [5]. Principal step of this method is based on reaction of readily
available a-tosyl substituted ureas or thioureas
6 with enolates of a-functionally substituted
ketones 7 (Scheme 1).
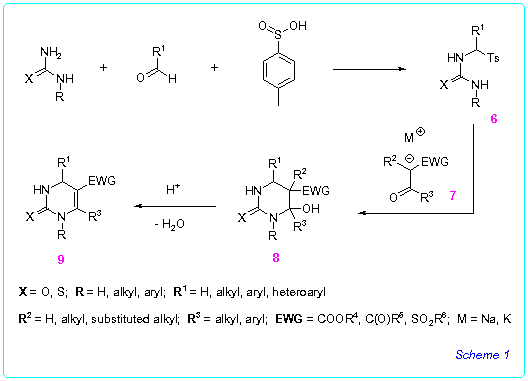
We showed that the method is very flexible and offers access to a large
variety of pyrimidines. We proposed that this approach could be applied
to the synthesis of hydrogenated pyrimidine-2-imines. Thus, a-tosyl
substututed guanidines were required for this synthesis. However, they
could not be prepared by direct three-component condensation of guanidine,
aldehydes and p-toluenesulfinic acid because of high basicity of
guanidine. That is why instead of guanidine we decided to use guanidines
bearing an electron-withdrawing group at nitrogen. We had in mind also
that this group should be removed in one of subsequent stages of the synthesis.
Thus, at the first time we used commercially available N-cyanoguanidine
10 (dicyandiamide) as starting compound. Here, we report the application
of this approach to the preparation of 5-functionally substituted 2-cyanimino-4-hydroxyhexahydropyrimidines
and 2-cyanimino-1,2,3,4-tetrahydropyrimidines.
 Results
and Discussion
Results
and Discussion
The desired a-tosyl substituted N-cyanoguanidines
11 were prepared by reaction of N-cyanoguanidine 10 with
aliphatic aldehydes (acetaldehyde, propionic aldehyde and butyraldehyde)
and p-toluesulfinic acid (water, r.t., 2-4 days) (Scheme 2).
The products 11a-c were isolated in 63-94 % yields by filtration
of the reaction mixtures. It should be noted that the reaction involves
only one of two unsubstituted nitrogen atoms of 10. The obtained
tosylguanidines 11a-c owing to their good purity were used for the
pyrimidine synthesis without further purification.

We found that 11a-c reacted readily (r.t., 6-7 h) with potassium
enolates of 1,3-dicarbonyl compounds (acetylacetone and benzoylacetone)
generated in situ by treatment of the corresponding CH-acids with
KOH in ethanol to give the corresponding 5-acyl-2-cyanimino-4-hydroxyhexahydropyrimidines
12 in 72-91 % yields. Analogously, ethyl 2-cyanimino-4-hydroxyhexahydropyrimidine-5-carboxylates
13 were prepared in 51-80 % yields starting from 11a-c and
b-oxoesters (ethyl
acetoacetate and ethyl butyrylacetate) (Scheme 3). The pyrimidines
12, 13 were formed in good diastereomeric purity.

Reaction of 11b with potassium enolate of tosylacetone 14
(ethanol, r.t., 7.5 h) gave rather unusual result. Instead of 15
we obtained a mixture of 15 and 16 in the ratio of 3:1 (Scheme
4). Probably, formation of 16 can be explained by equilibrium
of enolates A and B. Clearly, despite huge predominance of
A over B in the equilibrium, reaction rate of 11b
with B is much higher than with A because of steric and electronic
factors.

The obtained 2-cyanimino-4-hydroxypyrimidines 12, 13,
15, 16 can be easily dehydrated in the presence of acids
to produce the corresponding 2-cyanimino-1,2,3,4-tetrahydropyrimidines
17-20. Really, refluxing 13b and TsOH (0.2 equiv.) in ethanol
for 1.2 h gave the tetrahydropyrimidine 18b in 73 % yield. Analogously,
a mixture of 19 and 20 in the ratio of 3:1 was prepared starting
from the mixture of 15 and 16 (3:1) (Scheme 5). The
pyrimidine 20 was easily separated by recryctallization from ethanol.

Mainly, however, 2-cyanimino-1,2,3,4-tetrahydropyrimidines 17,
18 were synthesized by convenient one-pot procedure starting directly
from a-tosyl substituted N-cyanoguanidines 11a-b.
According to this procedure, 11a-c reacted (r.t., 6-7 h) with potassium
enolates of 1,3-dicarbonyl compounds or b-oxoesters
to afford 12, 13 which without isolation were dehydrated
after addition of TsOH (0.2 equiv.) to the reaction mixtures and subsequent
refluxing for 1-2 h to afford 17, 18 in 46-73 % overall yields
(Scheme 6).

The prepared 2-cyaniminopyrimidines 12, 13, 15-20
can serve as starting compounds for syntheses of other 2-iminopyrimidines.
For example, we found that 5-acetyl-4-hydroxypyrimidines 12 (R1
= Me) in aq. KOH at r.t. give 4-hydroxypyrimidines 21 (27-82 % yields)
in result of removing the acetyl group in 12 (Scheme 7).
Probably, this transformation proceeds via the retro-Claisen reaction
in the acyclic isomeric form of 12.

 Conclusion
Conclusion
Thus, we have developed a new convenient method for the synthesis of hydrogenated
2-cyaniminopyrimidines using reaction of readily available a-tosyl
substituted N-cyanoguanidines with enolates of 1,3-dicarbonyl compounds
or b-oxoesters.
The obtained pyrimidines can serve as starting compounds in syntheses of
a large number of multifunctional 2-iminopyrimidines. The application of
the proposed method to the synthesis of other hydrogenated 2-iminopyrimidines,
including heterocyclic guanidine natural alkaloids and their analogs, is
currently in progress.
 References
References
1. Berlinck, R.G.S. Fortschr. Chem. Org. Naturst., 1995,
66, 119.
2. Berlinck, R.G.S. Nat. Prod. Rep., 1996, 13,
377.
3. Berlinck, R.G.S. Nat. Prod. Rep., 1999, 16,
339.
4. Hannon, C.L.; Anlyn, E.V. in Bioorganic Chemistry Frontiers,
ed. H. Dugas, Springer-Verlag, Berlin, Heidelberg, 1993, 3,
193.
5. Shutalev, A.D.; Kishko, E.A.; Sivova, N.V.; Kuznetsov, A.Yu.
Molecules 1998, 3, 100; Shutalev, A.D.; Kuksa, V.A.
Khim. Geterotsikl. Soedin. 1997, 105; Shutalev, A.D.; Kuksa,
V.A. Khim. Geterotsikl. Soedin. 1995, 97.
All comments on this poster should be sent by e-mail to (mailto:[email protected]
ona.edu)
[email protected]
with A0073 as the message subject of your e-mail.
![]() Introduction
Introduction
![]() Results
and Discussion
Results
and Discussion
![]() Conclusion
Conclusion
![]() References
References







