[A0077]
Synthetic Methods to Ferrocenomesogens containing a Pendent Ferrocenyl GroupChristopher Imrie*, Christa Loubser, Pieter Engelbrecht, Cedric W. McCleland, Nomfuneko Tolom and Vincent O. Nyamori
Department of Chemistry, University of Port
Elizabeth, PO Box 1600, Port Elizabeth 6000, Republic of South Africa
E-mail: [email protected]
Received: 4 August 2000 / Uploaded: 5 August 2000
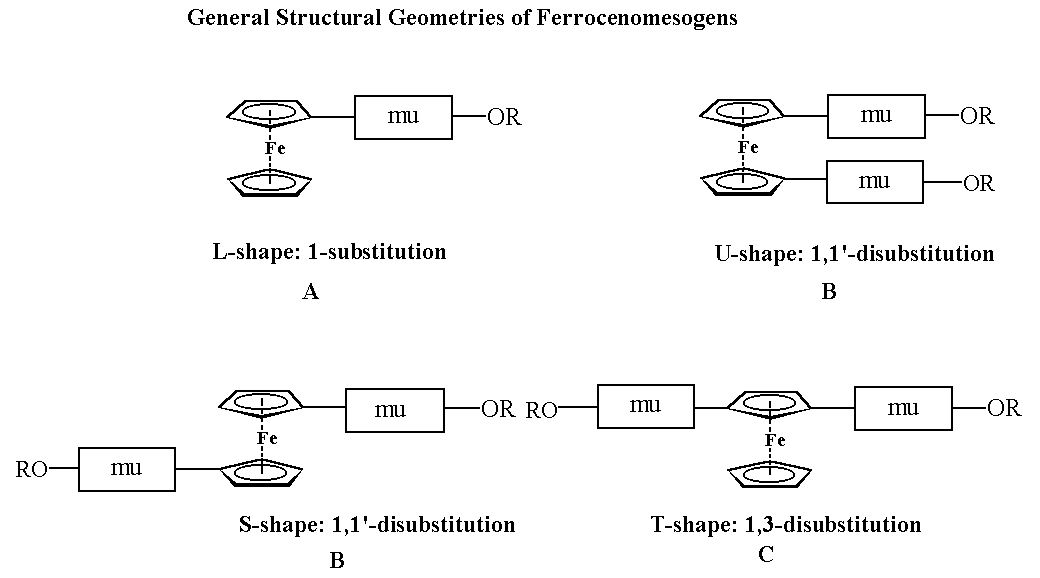
Ferrocene behaves much like a three dimensional arene and is useful for incorporating a metal atom into a molecule that requires a certain degree of structural rigidity such as a liquid crystal.1 The ferrocenyl unit is not only useful for modifying the shape of a molecule; in addition the metal atom with its large electron density can be used to modify the physical properties such as colour, polarisability and magnetism. Following the publication in 1976 of the first examples of ferrocene derivatives with liquid-crystalline properties (type A) 2and more notably since 1988 when the first liquid crystals based on a disubstituted ferrocene were synthesized (type B),3 there has been a growing interest in these compounds amongst chemists. The majority of ferrocenyl-containing liquid crystals or ferrocenomesogens (as we have named them) prepared so far belong to one of the structural types A, B or C. Although the synthesis of derivatives with interesting new shapes continue to be exciting, a systematic approach to structure-liquid crystal activity which will allow meaningful predictions has become necessary.

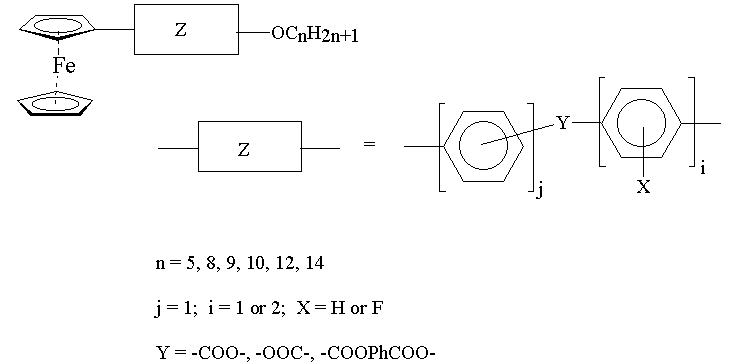
We have recently reported the use of a modified Suzuki cross-coupling procedure for the synthesis of 4-substituted phenylferrocenes.6 Iodoferrocene was reacted with a series of 4-substituted phenylboronic acids in the presence of a base and catalyst to provide a range of 4-substituted phenylferrocenes. By replacing the iodoferrocene with 4-bromophenylferrocene, the same reaction provides 4-substituted ferrocenylbiphenyl compounds. The isolated yields of the ferrocenylbiphenyl products were good-to-excellent in most cases (Table 1). Experience in the previous Suzuki work on substituted phenylferrocenes had shown that yields could be improved significantly by heating reactions involving electron-deficient arylboronic acids. The result in Table 1 (entry 5) supports that conclusion.
Table 1. Yield of ferrocenylbiphenyl compounds from the reaction of 4-bromophenylferrocene with arylboronic acids
Entry |
X |
4-Bromophenylferrocene (mmol) |
Time (days) |
Yield (%)a |
1 |
4-OCH3b |
1.5 |
14 |
93 |
2 |
4-CH3b |
1.5 |
14 |
88 |
3 |
4-OC8H17b |
1.5 |
14 |
65 |
4 |
4-Phb |
1.5 |
14 |
79 |
5 |
4-CHOc |
3.0 |
21 |
50 |
6 |
4-CHOb |
3.0 |
32 |
13 |
7 |
4-CHOb |
3.0 |
1 |
10 |
8 |
4-Fb |
3.0 |
14 |
89 |
9 |
4-COCH3c |
3.0 |
14 |
14 |
10 |
4-CH2Phc |
1.5 |
14 |
70 |

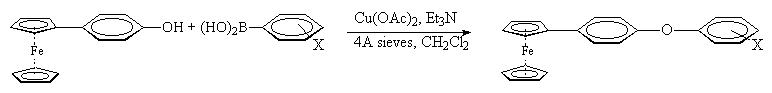
Substituent X |
Yield (%)a |
4-CH3b,c |
49 |
4-OCH3b,c |
41 |
3,4,5-(OCH3)3b,c |
42 |
4-OCH2Phb,c |
46 |
4-Hb,c |
51 |
4-OC8H17b,c |
21 |
4-Fb,c |
34 |
3-Fb,c |
27 |
4-Brb,c |
19 |
4-COCH3b,c |
32 |
4-CHOb,c |
26 |
4-CHOb,d |
34 |
4-OCF3b,c |
28 |
4-CF3b,c |
2 |
3-NO2b,d |
10 |
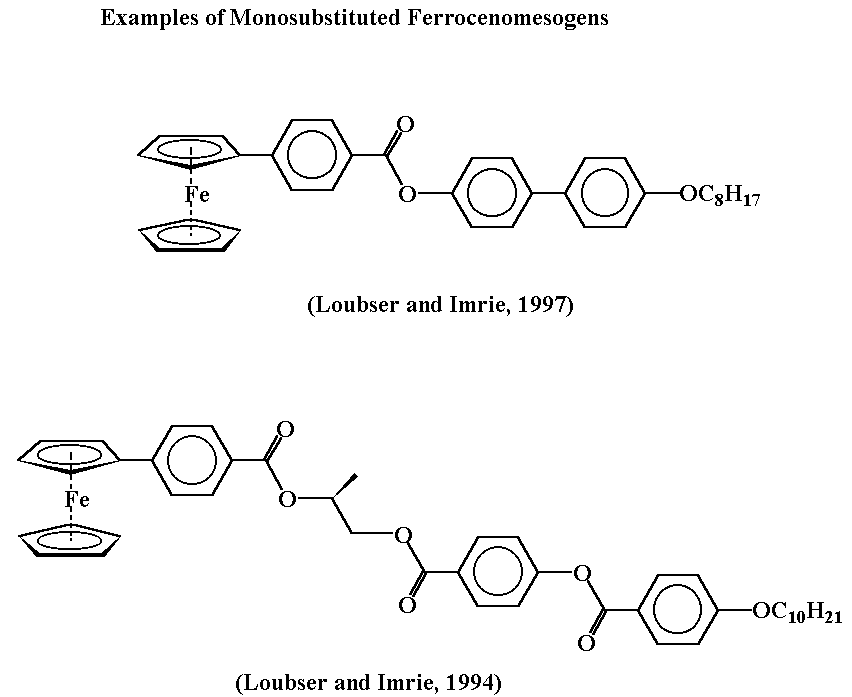
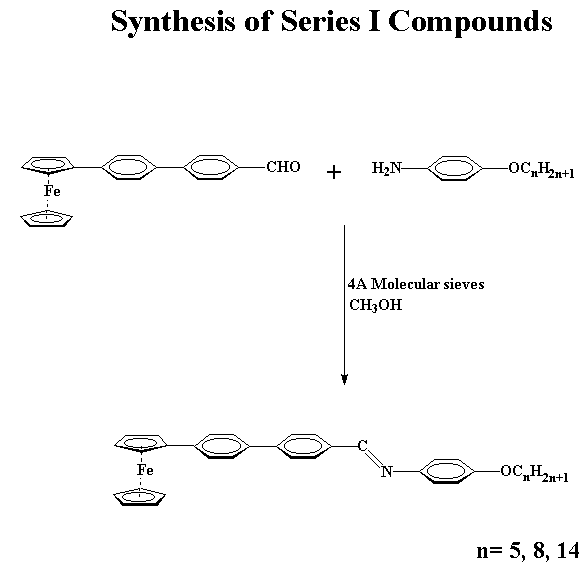
One of the objectives of the work was to observe the effect of introducing the ferrocenylbiphenyl- and ferrocenylphenylaryl ether unit into ferrocenomesogens. With this goal in mind, a series of Schiff bases, series I and series II were synthesized. The compounds of series I were synthesized by the reaction of 4'-formyl-4-biphenylferrocene with a series of n-alkoxyanilines. The compounds where n=8 and n=14 exhibit enantiotropic smectic and nematic phases, whereas the compound where n=5 exhibits a monotropic nematic phase. The liquid crystal domains in all cases are relatively short and the usual effect of chain lengthening in relation to the lowering of clearing temperatures is observed. Replacement of 4'-formyl-4-biphenylferrocene by 4-(4-formyl)phenoxyphenylferrocene in the reactions with the n-alkoxyanilines provided the corresponding Schiff base derivatives of series II in good yield. The compounds where n=5 and n=14 were prepared and in both cases, they exhibit normal melting behaviour.
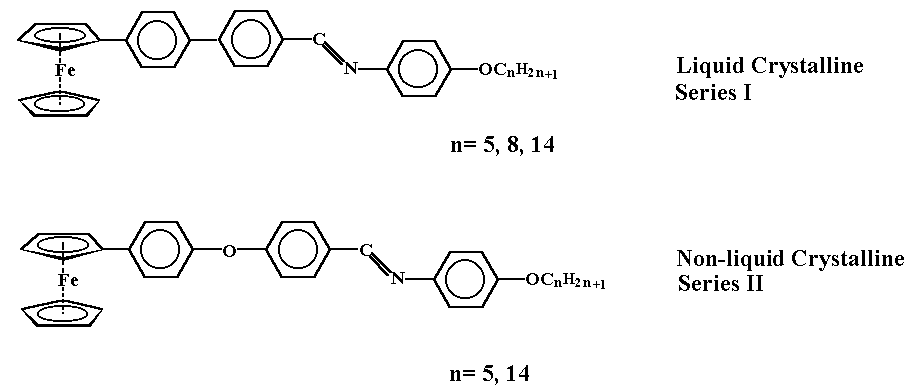
Long rigid molecules containing bulky pendent ferrocenyl moieties are useful for stabilizing nematic liquid crystal phases. Small alterations to the molecules which give rise to kinks in the molecular structure inhibit liquid-crystalline behaviour. Further work is in progress to establish other factors that could affect the liquid crystal properties of monosubstituted ferrocenomesogens. Modern synthetic methods are being used for the elaboration of the ferrocenyl-phenyl group.
References
1. R. Deschenaux and J.W. Goodby, in Ferrocenes, Homogeneous Catalysis, Organic Synthesis, Materials Science, eds. A. Togni and T. Hayashi, VCH, Weinheim, 1995, ch.9.
2. J. Malthete and J. Billard, Mol. Cryst. Liq. Cryst., 1976, 34, 117.
3. J. Bhatt, B.M. Fung, K.M. Nicholas and C.-D. Poon, J. Chem. Soc., Chem. Commun., 1988, 1439.
4. (a) C. Loubser and C. Imrie, J. Chem. Soc., Perkin Trans. 2, 1997, 399; (b) C. Imrie and C. Loubser, J. Chem. Soc., Chem. Commun., 1994, 2159; (c) C. Loubser, C. Imrie and P.H. Van Rooyen, Adv. Mater., 1993, 5, 45.
5. (a) Yu.G. Galyametdinov, O.N. Kadkin and I.V. Ovchinnikov, Izv. Akad. Nauk SSSR, Ser. Khim., 1990, 2462 (Engl. Transl., Bull. Acad. Sci. USSR, Div. Chem. Sci., 1990, 39, 2235); (b) T. Hanasaki, M. Ueda and N. Nakamura, Mol. Cryst. Liq. Cryst., 1993, 237, 329; (c) Yu.G. Galyametdinov, O.N. Kadkin and A.V. Prosvirin, Izv. Akad. Nauk SSSR, Ser. Khim., 1994, 941 (Engl. transl. Bull. Acad. Sci. USSR, Div. Chem. Sci., 1994, 43, 887); (d) Yu.G. Galyametdinov, O.N. Kadkin, V.I. Gavrilov and L.M. Tinchurina, Izv. Akad. Nauk SSSR, Ser. Khim., 1995, 358.
6. C. Imrie, C. Loubser, P. Engelbrecht and C.W. McCleland, J. Chem. Soc., Perkin Trans. 1, 1999, 2513.
7. F. Theil, Angew. Chem. Int. Ed., 1999, 38, 2345.
8. D.A. Evans, J.L. Katz and T.R. West, Tetrahedron Lett., 1998, 39, 2937.
9. D.M.T. Chan, K.L. Monaco, R.-P. Wang and M.P. Winters, Tetrahedron Lett., 1998, 39, 2933.
All comments on this poster should be sent by e-mail to (mailto:[email protected]) [email protected] with A0077 as the message subject of your e-mail.