[A0084]
A Chiral Silyl Ether as Auxiliary for the Asymmetric Nucleophilic Addition to a- and b-Silyloxy Carbonyl Compounds
Michael Trzoss, Stefan Bienz
Org.-chem. Institut, Universität Zürich,
Winterthurerstrasse 190, 8057 Zürich
E-mail: [email protected], [email protected]
URL: http://www.unizh.ch/oci/group.pages/bienz/index.html
Received: 9 August 2000 / Uploaded: 16 August
Introduction
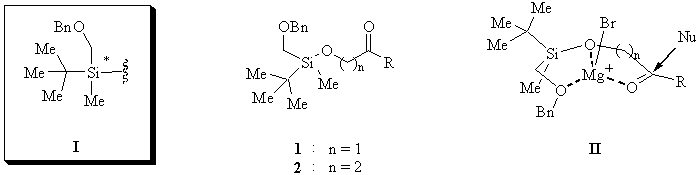
Silyl ethers are well established protective groups in organic synthesis [1]. They are easily prepared and cleaved, and their reactivity can readily be controlled by the appropriate choice of the groups attached to silicon. In the course of our ongoing investigation of the chiral (tert-butyl)(methyl)(benzyloxymethyl)silyl group (I) as an auxiliary to control stereoselective processes [2] we got interested in the combined use of I as a protective and stereochemically directing group.

Figure 1
For an initial investigation, we envisioned racemic silyl ethers of a- and b-hydroxyketones (compounds of type 1 and 2, Figure 1) as suitable substrates to study the stereodirecting effect of the ether-linked chiral silyl moiety on the MgBr2-mediated nucleophilic addition of Grignard reagents to carbonyl groups. Tridentate chelate transition structures of type II were expected to effect efficient chirality transfer from the silicon center to the carbon framework.
Results and Discussion
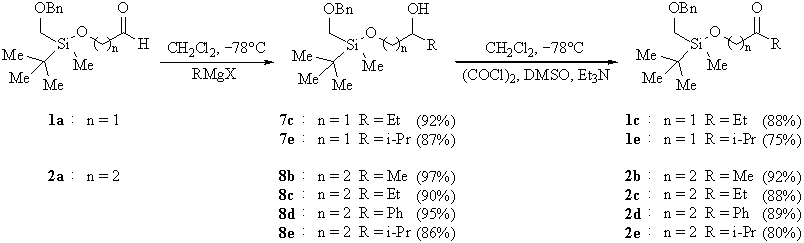
A series of silylated compounds of type 1 and 2, the starting materials for our investigation, were prepared from readily available racemic chlorosilane 3 [3]. Direct silylation of commercial a-hydroxyketones 4b,d afforded compounds 1b,d. Silylation of 1,2-diol 5a and 1,3-diols 6a,b followed by oxidation gave rise to compounds 1a and 2a,b, respectively (Scheme 1).

Scheme 1
Substrates 1c,e and 2b�e were obtained by the addition of the appropriate Grignard reagent to aldehyde 1a resp. 2a and subsequent oxidation (Scheme 2).
Scheme 2
The stereoselective addition reactions summarized in Table 1 were performed as follows: silyl ethers 1a�e or 2a�e were precomplexed at �78�C in CH2Cl2 with 5 equivalents of MgBr2 and 3 equivalents of the Grignard reagent were added. Reactions were quenched after approximately 2 h and worked up by extraction followed by chromatography. The yields of the addition products were generally high (80�95%), and the ratios of the diastereomeric products 9�31 and 9��31� were determined by 1H-NMR spectroscopy on the crude mixtures (before chromatography).
Table 1
|
Entry |
n |
Start. Material No. |
R1 |
R2MgX |
Produkt No. |
dr |
|
1 |
1 |
1a |
H |
MeMgBr |
9 / 9� |
66 : 34 |
|
2 |
1 |
1a |
H |
EtMgBr |
10 / 10� |
60 : 40 |
|
3 |
1 |
1a |
H |
PhMgBr |
11 / 11� |
59 : 41 |
|
4 |
1 |
1a |
H |
i-PrMgCl |
12 / 12� |
53 : 47 |
|
5 |
1 |
1a |
H |
t-BuMgCl |
13 / 13� |
50 : 50 |
|
6 |
1 |
1b |
Me |
PhMgBr |
14 / 14� |
76 : 24 |
|
7 |
1 |
1c |
Et |
PhMgBr |
15 / 15� |
72 : 28 |
|
8 |
1 |
1e |
i-Pr |
PhMgBr |
16 / 16� |
53 : 47 |
|
9 |
1 |
1d |
Ph |
MeMgBr |
17 / 17� |
71 :29 |
|
10 |
1 |
1d |
Ph |
EtMgBr |
18 / 18� |
66 : 34 |
|
11 |
1 |
1d |
Ph |
i-PrMgCl |
19 / 19� |
64 : 34 |
|
12 |
1 |
1d |
Ph |
t-BuMgCl |
20 / 20� |
52 : 48 |
|
13 |
2 |
2a |
H |
MeMgBr |
21 / 21� |
43 : 57 |
|
14 |
2 |
2a |
H |
EtMgBr |
22 / 22� |
37 : 63 |
|
15 |
2 |
2a |
H |
PhMgBr |
23 / 23� |
34 : 66 |
|
16 |
2 |
2a |
H |
i-PrMgCl |
24 / 24� |
36 : 64 |
|
17 |
2 |
2b |
Me |
PhMgBr |
25 / 25� |
13 : 87 |
|
18 |
2 |
2c |
Et |
PhMgBr |
26 / 26� |
16 : 84 |
|
19 |
2 |
2e |
i-Pr |
PhMgBr |
27 / 27� |
37 : 63 |
|
20 |
2 |
2d |
Ph |
MeMgBr |
28 / 28� |
25 : 75 |
|
21 |
2 |
2d |
Ph |
EtMgBr |
29 /29� |
18 : 82 |
|
22 |
2 |
2d |
Ph |
i-PrMgCl |
30 /30� |
20 : 80 |
|
23 |
2 |
2d |
Ph |
t-BuMgCl |
31 / 31� |
20 : 80 |
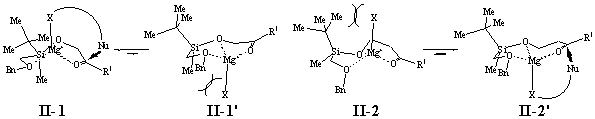
Several trends can be extracted from the results presented in Table 1. (1) It is obvious that p-facial selectivity under the influence of the group I is not very high and different for the two types of substrates: compounds of type 1 permit preferred lk- and substrates of type 2 ul-attacks of the nucleophiles. (2) Highest selectivities were obtained with compounds of type 2, compound of type 1 showing overall lower selectivities. (3) With the exception of the aldehydes 1a and 2a, increase of bulkiness of the R1 group for both types of substrates is accompanied by a decrease of diastereofacial preference of the attacks, and (4) increase of the size of the nucleophiles results in decreased selectivity for compounds 1 and increased selectivity for compounds 2.
Scheme 3
The results can be explained to a great extent by scrutinizing the proposed chelate structures II-1/II-1� and II-2/II-2� for the two types of substrates and sites of attack. Simple molecular models and semi-empirical calculations (PM3, MacSpartan) suppose II-1 for compounds 1 and II-2� for compounds 2 to be the thermodynamically favored complexes, thus explaining the different p-facial selectivities for the two different substrates (Scheme 3). Both complexes II-1 and II-2� are rather encumbered structures. Increase of steric strain by the incorporation of larger R1 groups is thus supposed to decrease the stability of both these complexes, favoring competitive �open-chain-controlled� processes, and accounting for the observed decrease of stereoselectivity (Entries 6�8 and 17�19). Increase of the size of R2 of the Grignard reagent, on the other hand, would affect the stability of related compexes of type II-1 (Entries 9�12) more than those of complexes of type II-2� (Entries 20�23). As a consequence of the proximity of the residues at the Mg-atom to the tert-butyl group at the Si-atom in complexes of type II-1, their stability would be strongly decreased by the increase of the size of R2. This is expected to be lesser the case in complexes of type II-2, where the residues of the organometal is neighbored by the rather small methyl group only. Thus, the observed stereochemical effects accompanied by the increase of the size of R2 in the organometallic reagent can be explained by the increasing importance of �open-chain-controlled� processes in the case of compounds 1 and by enhanced steric discrimination in the case of compounds 2.
With the above results at hand it is readily understood that the addition of Grignard reagent 32 to a-silyloxyketone 1b leads with only low stereoselectivity to the alcohols 33/33�, precursors of frontalin (34) the attracting pheromone of the pine beetle [4] (Scheme 4).

It is not surprising, on the other hand, that the addition of the Grignard reagent 35 of a sterically demanding ortho-substituted benzene derivative to b-silyloxyketone 2b led to the respective addition products 36/36� with high stereoselectivity (dr 92:8). Compounds 36/36� � hydrolyzed and subsequently oxidized at the primary alcohols, followed by acid catalyzed tandem-cyclization � afforded 37, whose derivatives have shown interesting physiological properties due to their structural similarity to Isocannabinol 38. (Scheme 5) [5].
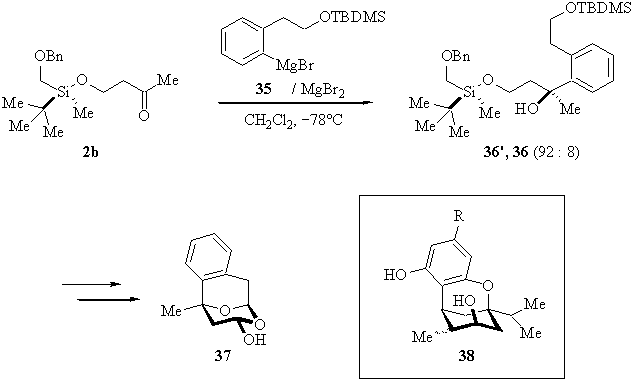
Scheme 5
Conclusion
In conclusion it is shown with the above investigation that chiral silicon groups, attached to a prostereogenic functionality by means of an ether linkage, can act in principal � at least in specific cases � efficiently as stereochemical directors. The potential of this principle might be increased by the structural optimization of the auxiliary group, which has not been performed yet.
References
[1] P. J. Kocienski, Protecting Groups, Georg Thieme Verlag Stuttgart, New York 1994.
[2] S. Bienz, Chimia 1997, 51, 133.
[3] A. Chapeaurouge, S. Bienz, Helv. Chim. Acta 1993, 76, 1876.
[4] G. W. Kinzer, A. F. Fentiman, T. F. Page, R. L. Folz, J. P. Vite, G. B. Pitman, Nature (London) 1969, 221, 477.
[5] B. Wünsch, G. Bauschke, Liebigs Ann. Chem. 1992, 345.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0084 as the message subject of your e-mail.