[A0088]
Chemistry of Substituted Quinolinones. V. Synthesis and Utility of Quinolinylphosphazenes in Amination of 8-Methylquinoline
Mostafa M. Ismail, Mohamed Abass and Mohamed M. Hassan
Department of Chemistry, Faculty of Education, Ain Shams University,
Roxy, Cairo 11711, Egypt
E-mail: [email protected]
Received: 15 August 2000 / Uploaded: 20 August
Abstract: 2,4-Dihalo-8-methylquinolines 2,3 have been prepared and subjected to azidation and hydrazination reactions showing interesting regioselective properties to give compounds 4-10. The targeted aminoquiolines 13, 14, 15, 17, 19, 22 and 23 were obtained via hydrolysis of their corresponding phosphazenes 11, 12, 16, 18, 20 and 21, respectively. These phosphazenes have been preliminary obtained by condensation of azido and/or tetrazoloquinolines 4, 5, 6, 10, 18 and 22, with triphenylphosphine in either boiling benzene or 1,2-dichlorobenzene.
Keywords: haloquinolines, hydrazinoquinolines, tetrazoloquinolines, azidoquinolines, phosphazenes, aminoquinolines
Introduction
In continuation to our current research work on substituted quinolines and quinolin-2-ones [1-3], this article deals with study of synthesis and nucleophilic substitution of 2,4-dihalo-8-methylquinolines. Direct nucleophilic amination of haloquinolines is mostly not efficient in production of aminoquinolines. Herein the known Staudinger method was used for reduction of azido and/or tetrazoloquinolines, which were obtained from haloquinolines, to the desired aminoquinolines [4-7].
Results and Discussion
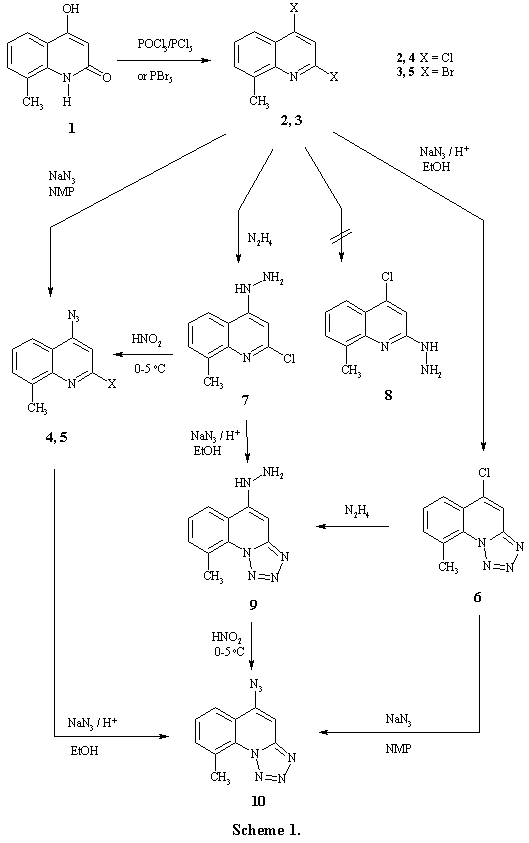
4-Hydroxy-8-methyl-1,2-dihydroquinolin-2-one (1) [3] was subjected to chlorination reaction using a mixture of phosphorus oxychloride and phosphorus pentachloride at the molar ratio (3:2). Using of phosphorus oxychloride gave a very low yield of 2,4-dichloro-8-methylquinoline (2), while phosphorus pentachloride lonely yielded a tary product that needs several crystallizations to obtain a much lesser yield of dichloroquinoline 2. On the other hand phosphorus pentabromide was used to obtain 2,4-dibromo-8-methylquinoline (3). Even the yield herein is also low somewhat but trials to enhance this yield, by increasing time of reaction or amount of brominating agent or addition of phosphorus oxybromide or phosphorus tribromide, met no success and mostly effected production of several by-products. The azidation reaction of both dihaloquinolines 2 and 3 was investigated to check their behaviour towards nucleophilic substitution at both a� and g�positions. Thus, when compounds 2 and/or 3 were reacted with sodium azide at the molar ratio (1:1), in DMF or N-methylpyrrolidone (NMP), only 4-azido-2-halo-8-methylquinolines 4,5 were afforded while the expected 5-chloro-9-methyl[1,2,3,4]tetrazolo[1,5-a]quinoline (6) had not been achieved at these conditions. This result is simply concluded by the findings in literature in which kinetic studies indicate that g-chloro atom of dichloquinolines is about two times more reactive towards nucleophiles and predominately an addition-elimination process occurred [8,9]. This led us to try the latter reaction in different conditions. Thus it was found that addition of an acid as a catalyst led to the tetrazoloquinoline 6. This reaction was found catalyzable by methane sulphonic acid or trichloroacetic acid, or trifluoroacetic acid in either ethanol or dioxane. The best yield was obtained on using toluene-4-sulphonic acid in absolute ethanol.

Hydrazination of compound 2 showed the same interesting regioselective property that observed towards azidation. Reaction of the dichloroquinoline 2 with hydrazine hydrate furnished only 2-chloro-4-hydrazino-8-methylquinoline (7). It is clear that there is a possibility in this reaction to form the isomeric 2-hydrazinoquinoline derivative 8. Since IR and 1H NMR spectra of the product of this reaction may not satisfactorily enough for the elucidation of which isomer was actually obtained, two reactions were carried out with compound 7. Treatment of which with nitrous acid led to the formation of 4-azido-2-chloro-8-methylquinoline (4), that was previously obtained and characterized. Also, treating compound 7 with sodium azide in the presence of toluene-4-sulphonic acid gave the same product obtained by reaction of 5-chlorotetrazolo-quinoline 6 with hydrazine hydrate which its structure was indicated and characterized as 5-hydrazino-9-methyl[1,2,3,4]tetrazolo[1,5-a]quinoline (9).
5-Azido-9-methyl[1,2,3,4]tetrazolo[1,5-a]quinoline (10) was achieved through different pathways. Treating compound 2 with high excess of sodium azide and refluxing for long time was found not benefit to obtain compound 10 in good yield or satisfactory purity where the compound 4 was the main product beside other different by-products. It is thought judicious that step-wise treatment of the dihaloquinolines 2,3 with sodium azide might furnish the desired product. So that compounds 4 and/or 5 were treated with sodium azide in the presence of an acid catalyst and on the other hand compound 6 was treated with sodium azide, in boiling DMF, to give the target compound 10. Moreover when, hydrazinotetrazoloquinoline 9 was reacted with nitrous acid at 0-5 oC, the azidotetrazoloquinoline 10 was also obtained (Scheme 1).
The eighty years�old Staudinger method for reduction of azides into their corresponding amines was utilized to obtain the desired aminoquinolines [4,5,7,10]. The method includes reaction of various azides with triphenylphosphine to give the phosphazene derivatives followed by hydrolysis of which with aqueous hydrochloric acid leading to the aminoquinoline derivatives. Thus, azides 4 and/or 5 were reacted with triphenylphosphine in boiling benzene to give the 4-quinolinylphosphazenes 11 and 12. Hydrolysis of the latter phosphazenes by dilute hydrochloric acid gave a mixture of 4-amino-2-halo-8-methylquinolines 13 or 14 and 4-amino-8-methyl-1,2-dihydroquinolin-2-one (15).
On carrying out condensation reaction of tetrazoloquinoline 6 with triphenylphosphine, in boiling benzene, the reactants were completely recovered unchanged. This reaction was found successful on using a much higher boiling point solvent such as DMSO or 1,2-dichlorobenzene. The latter solvent led to a much better yield of 4-chloro-2-quinolinylphosphazene 16.

Acid hydrolysis of phosphazene 16 furnished 2-amino-4-chloro-8-methylquinoline (17). Reacting compound 16 with sodium azide in DMF resulted in 4-azido-2-quinolinylphosphazene 18 which upon hydrolysis gave the same 2-amino-4-azido-8-methylquinoline (19) that afforded from azidation of compound 17.
The difference in activity of both isomers 4 and 6 towards condensation with triphenylphosphine prompted our interest to study the behavior of azidotetrazoloquinoline 10 towards the same reagent. So that when compound 10 was subjected to react with an equimolar amount of triphenylphosphine, in boiling benzene, the tetrazoloquinolylphosphazene 20 was achieved which hydrolyzed ton give aminotetrazoloquinoline 22.
Use of excess reagent did not reveal any variation of the product category. This may be attributed due to thermal stability of tetrazole ring compared by azide group. In the meantime, replacement of benzene as the reaction solvent by 1,2-dichlorobenzene showed interesting results where the excess reagent led to another product that was characterized as the diphosphazene 21. It was very important to verify if both phosphazenes 18 and 20 could be converted to the diphosphazene 21 by the action of triphenylphosphine. This was investigated and also it was found that solvent of these reactions plays a role. Thus, compound 18 underwent smooth condensation with triphenylphosphine in dry benzene, while compound 20 needed 1,2-dichlorobenzene to furnish the diphosphazene 21. Finally the targeted 2,4-diamino-8-methylquinoline (23) was obtained by hydrolysis of the diphosphazene 21, using dilute hydrochloric acid (Scheme 2).
Experimental
General
Melting points were measured on a Gallen-Kamp MFB-595 in open capillary tubes and are incorrected. IR spectra were recorded on Perkin-Elmer 598 and FT�IR 1650 instruments (n , cm-1). 1H NMR spectra were measured on Jeol FX90�NMR or Varian EM-390 NMR (90 MHz) spectrometers (d, ppm) using DMSO-d6 or CDCl3 as solvents and TMS as internal standard. Elemental microanalyses were performed on a Perkin-Elmer CHN-2000 analyzer. Compound 1 was prepared according to literature [3]. Analytical and spectral data are listed in Tables 1 and 2, respectively.
Table 1. Analytical data of the new compounds
|
Cpd No |
Yield (%) |
M.P.(oC) Solvent |
Mol. Form. Mol. Wt. |
Elemental analysis Calcd./Found (%) |
||
|
C |
H |
N |
||||
|
2 |
46 |
81-82 MeOH |
C10H7Cl2N 212.08 |
56.64 56.70 |
3.33 3.39 |
6.60 6.60 |
|
3 |
23 |
62-64 EtOH |
C10H7Br2N 300.98 |
39.91 39.88 |
2.34 2.31 |
4.65 4.70 |
|
4 |
50a, 66b |
92 MeOH |
C10H7ClN4 218.65 |
54.93 54.90 |
3.23 3.24 |
25.62 25.61 |
|
5 |
78 |
155-156 EtOH |
C10H7BrN4 263.10 |
45.65 45.60 |
2.68 2.68 |
21.30 21.31 |
|
6 |
55 |
175 Pet. Ether |
C10H7ClN4 218.65 |
54.93 54.88 |
3.23 3.20 |
25.62 25.64 |
|
7 |
61 |
192-194 Benzene |
C10H10Cl N3 207.66 |
57.84 57.84 |
4.85 4.81 |
20.26 20.22 |
|
9 |
35 |
210 DMF/H2O |
C10H10N6 214.23 |
56.07 56.09 |
4.71 4.69 |
38.25 38.22 |
|
10 |
75a, 70b, 64c |
208 THF |
C10H7N7 225.21 |
53.33 53.30 |
3.13 3.10 |
43.53 43.61 |
|
11 |
80 |
196-197 EtOH |
C28H22ClN2P 452.93 |
74.25 74.24 |
4.90 4.91 |
6.19 6.20 |
|
12 |
85 |
245-246 EtOH |
C28H22BrN2P 497.38 |
67.61 67.59 |
4.46 4.40 |
5.63 5.60 |
|
13 |
65 |
170 DMF |
C10H9ClN2 192.65 |
62.35 62.30 |
4.71 4.70 |
14.54 14.58 |
|
14 |
42 |
> 300 DMF |
C10H9BrN2 237.10 |
50.66 50.58 |
3.83 3.82 |
11.82 11.78 |
|
15 |
32 |
>300 DMF |
C10H10N2O 174.20 |
68.95 69.00 |
5.79 5.74 |
16.08 16.00 |
|
16 |
70 |
224 EtOEt |
C28H22ClN4 452.92 |
74.25 74.23 |
4.90 4.90 |
6.19 6.20 |
|
17 |
70 |
>300 DMF |
C10H9ClN2 192.65 |
62.35 62.33 |
4.71 4.70 |
14.54 14.55 |
|
18 |
80 |
145-147 DMF/H2O |
C28H22N5P 459.49 |
73.19 73.20 |
4.83 4.81 |
15.24 15.32 |
|
19 |
60a, 74b |
220-222 DMF |
C10H9N5 199.22 |
60.29 60.31 |
4.55 4.52 |
35.16 35.24 |
|
20 |
65 |
168-170 Acetone |
C28H22N5P 459.49 |
73.19 73.25 |
4.83 4.81 |
15.24 15.30 |
|
21 |
75a, 81b, 85c |
136 Benzene |
C46H37N3P2 693.77 |
79.64 79.71 |
5.37 5.32 |
6.06 6.10 |
|
22 |
70 |
>300 DMF |
C10H9N5 199.22 |
60.29 60.32 |
4.55 4.52 |
35.16 35.21 |
|
23 |
70 |
>300 DMF |
C10H11N3 173.22 |
69.34 69.42 |
6.40 6.30 |
24.26 24.32 |
a)
, b), c) Yields (%) of methods [a], [b], and [c] respectively.2,4-Dichlro-8-methylquinoline (2)
A mixture of hydroxyquinolone 1 (0.1 mol), phosphorus oxychloride (0.3 mol) and phosphorus pentachloride (0.2 mol) was heated under reflux for 4 h, then left to cool to room temperature and poured into ice cold water. The white precipitate that formed was filtered off, washed with water, and crystallized.
2,4-Dibrmo-8-methylquinoline (3)
A mixture of hydroxyquinolone 1 (0.01 mol) and phosphorus pentabromide (0.03 mol) was heated under reflux for 1 h, then left to cool poured into ice cold water. The solid deposits were collected by filtration and crystallized.
4-Azido-2-chloro-8-methylquinoline (4)
[a] To a solution of dichloroquinoline 2 (0.01 mol) in NMP (50 ml), sodium azide (0.01) was added, then the mixture was warmed to 80-90oC for 2 h. Afterwards, the mixture was diluted with cold water (50 ml) to give a white precipitate, which was filtered off and crystallized.
[b] To a solution of compound 7 (0.01 mol) in hydrochloric acid (10 ml, 1 M), sodium nitrite solution (10 ml, 1 M) was added drop-wise with continuous stirring in an ice-bath at 0-5 oC. After completion of addition, the mixture was stirred for further 30 min, then filtered and the solid residue that obtained was recrystallized.
4-Azido-2-bromo-8-methylquinoline (5)
A solution of dibromoquinoline 3 (0.01 mol) in DMF (30 ml) was treated with sodium azide (0.01) and refluxed for 1 h. Then, the mixture was poured into ice-cold water and the precipitate so formed was collected by filtration and crystallized.
5-Chlor-9-methyl[1,2,3,4]tetrazolo[1,5-a]quinoline (6)
A mixture of dichloroquinoline 2 (0.01 mol), sodium azide (0.01 mol) and toluene-4-sulphonic acid (0.03 mol) in ethanol (30 ml) was heated under reflux on a boiling water- bath for 5 h. Afterwards, the mixture was poured into crushed ice and the precipitate so formed was filtered off and crystallized.
2-Chloro-4-hydrazino-8-methylquinoline (7)
To a sodium of dichloroquinoline 2 (0.01 mol), in ethanol (25 ml), hydrazine hydrate (0.03 mol) was added and the mixture was heated under reflux for 6 h. Then the reaction mixture was poured into crushed ice and the obtained precipitate was filtered off and crystallized.
5-Hydrazino-9-methyl[1,2,3,4]tetrazolo[1,5-a]quinoline (9)
[a] A mixture of chlorotetrazoloquinoline 6 (0.01 mol) and hydrazine hydrate (0.01 mol) in ethanol (25 ml) was refluxed for 1 h. The reaction mixture was then cooled and poured into ice-cold water. The solid so formed was collected by filtration and crystallized.
[b] A mixture of the compound 7 (0.01 mol), sodium azide (0.015 mol) and toluene-4-sulphonic acid (0.005 mol), in ethanol (30 ml), was refluxed on a boiling water-bath for 4 h. Then, the mixture was diluted with water (30 ml) and neutralized with sodium bicarbonate. The precipitate so obtained was filtered off and crystallized.
5-Azido-9-methyl[1,2,3,4]tetrazolo[1,5-a]quinoline (10)
[a] A mixture of either the 4-azido-2-haloquinoline 4 or 5 (0.01 mol) with sodium azide (0.015 mol) and toluene-4-sulphonic acid (0.005 mol), in ethanol (30 ml), was refluxed for 5 h. Then the mixture was diluted with cold water to give solid precipitate that was filtered off and crystallized.
[b] To a solution of compound 6 (0.01 mol) in DMF (20 ml), sodium azide (0.01 mol) was added, then the mixture was refluxed for 8 h and poured into cold water. The deposits were filtered off and crystallized.
[c] To a solution of compound 9 (0.01 mol) in hydrochloric acid (10 ml, 2 M), sodium nitrite (10 ml, 1 M) was added dropwise with continuous stirring in an ice bath at 0-5 oC. After completion of addition, the mixture was stirred for further 30 min, then filtered and the solid residue that obtained was recrystallized.
2-Halo-8-methyl-4-(triphenylphosphoranylideneamino)quinolines 11,12
A mixture of azidoquinolines 4 and/or 5 (0.01 mol), triphenylphosphine (0.011 mol) and benzene (25 ml) was refluxed for 3h. Then the excess solvent was evaporated in vacuum and the solid deposits so obtained was triturated with petroleum ether (40-60 oC), filtered off and crystallized.
Hydrolysis of Phosphazenes 11 and 12
The phosphazenes 11 and/or 12 (0.01) was treated with hydrochloric acid (50 ml, 6 M) and refluxed for 3 h. then left to cool and filtered. The clear filtrate was neutralized using aqueous sodium bicarbonate solution. The precipitate so formed was collected by filtration and crystallized from DMF (35 ml) to give the aminoquinolone 15.Concentration of the crystallization mother liquor to half of its initial volume (~ 15 ml) furnished aminoquinolines 13 and/or 14, respectively.
4-Chlor-8-methyl-2-(triphenylphosphoranylideneamino)quinoline (16)
A mixture of tetrazoloquinoline 6 (0.01 mol), triphenylphosphine (0.01 mol) and 1,2-dichlorobenzene (30 ml) was heated under reflux for 6 h. The solvent was evaporated in vacuum and the residue was triturated with diethyl ether (20 ml). The solid so obtained was filtered off, washed with excess diethyl ether (100 ml), and crystallized.
Table 2. Spectral data of the New Compounds.
|
Cpd No |
IR, n (cm-1) |
1 H NMR, d (ppm) |
|
2 |
1615 (C=N) , 760 (C-Cl) |
7.85-7.20 (m, 3H, arom), 6.30 (s, 1H, 3-H), 2.60 (s, 3H, CH3) |
|
3 |
1610, (C=N), 760, 645 (C-Br) |
8.05-7.15 (m, 3H, arom), 5.85 (s, 1H, 3-H), 2.55 (s, 3H, CH3) |
|
4 |
2120 (N3), 1608 (C=N), 762 (C-Cl) |
7.85-7.10 (m, 3H, arom), 6.85 (s, 1H, 3-H), 2.60 (s, 3H, CH3) |
|
5 |
2115 (N3), 1610 (C=N), 670 (C-Br) |
7.67-7.14 (m, 3H, arom), 6.86 (s, 1H, 3-H), 2.50 (s, 3H, CH3) |
|
6 |
1605, (C=N), 1100, 1080, 1040 (tetrazole) |
7.86-7.56 (m, 3H, arom), 6.92 (s, 1H, 3-H), 2.32 (s, 3H, CH3) |
|
7 |
3450, 3340, 3270 (NH2, NH), 1630 (C=N) and 745 (C-Cl) |
8.20 (bs, 1H, N-H), 7.95-7.15 (m, 3H, arom), 6.10 (s, 1H, 3-H), 4.40 (s, 2H, NH2), 2.55 (s, 3H, CH3) |
|
9 |
3370, 3280 (NH2,NH), 1610 (C=N), 1100-1060 (tetrazole) |
8.30 (bs, 1H, N-H), 7.96-7.15 (m, 3H, arom), 6.30 (s, 1H, 3-H), 4.30 (s, 2H, NH2), 2.90 (s, 3H, CH3) |
|
10 |
2120 (N3), 1605 (C=N), 1100, 1080, 1040 (tetrazole) |
7.95-7.15 (m, 3H, arom), 6.80 (s, 1H, 3-H), 2.80 (s, 3H, CH3) |
|
11 |
1630 (C=N), 1430 (P=N), 720 (C-Cl) |
8.05-7.10 (m, 18H, arom), 5.90 (s, 1H, 3-H), 2.40 (s, 3H, CH3) |
|
12 |
3060, 1615 (C=N), 1440 (P=N), 670 (C-Br) |
8.05-7.10 (m, 18H, arom), 6.95 (s, 1H, 3-N), 2.55 (s, 3H, CH3) |
|
13 |
3490, 3400-3220 (NH2), 1625 (C=N), 745, 690 (C-Cl) |
7.85-7.15 (m, 3H, arom), 6.30 (s, 2H, NH2), 5.85 (s, 1H, 3-H), 2.45 (s, 3H, CH3) |
|
14 |
3430, 3320 (NH2), 1620 (C=N), 660 (C-Br) |
7.95-7.15 (m, 3H, arom), 6.25 (s, 2H, NH2), 5.95 (s, 1H, 3-H), 2.30 (s, 3H, CH3) |
|
15 |
3430, 3388, 3200 (NH2, NH), 1660 (C=O) |
10.20 (bs, 1H, CONH), 8.10-7.00 (m, 3H, arom), 6.20 (s, 2H, NH2), 5.80(s, 1H, 3-H), 2.30 (s, 3H, CH3) |
|
16 |
1610 (C=N), 1445 (P=N), 720 (C-Cl) |
8.04-7.47 (m, 17H, arom), 7.09 (s, 1H, 3-H), 6.91 (d, 1H, 7-H), 2.53 (s, 3H, CH3) |
|
17 |
3480, 3330, 3200 (NH2), 1625 (C=N), 740 (C-Cl) |
8.15 (d, 1H, 5-H), 7.55-7.40 (m, 2H, 6-H + 7-H), 6.69 (s, 1H, 3-H), 4.61 (s, 2H, NH2), 2.79 (s, 3H, CH3) |
|
18 |
2130 (N3), 1620 (C=N), 1480, 1445 (P=N) |
8.06-7.10 (m, 17H, arom), 6.71 (d, 1H, 7-H), 6.35 (s, 1H, 3-H), 2.53 (s, 3H, CH3) |
|
19 |
3400, 3340 (NH2), 2120 (N3), 1620 (C=N) |
8.05-7.15 (m, 3H, arom), 6.35 (s, 2H, NH2), 5.90 (s, 1H, 3-H), 2.35 (s, 3H, CH3) |
|
20 |
1608 (C=N), 1485, 1440 (P=N), 1100,1020 (tetrazole) |
7.95-7.15 (m, 18H, arom), 6.35 (s, 1H, 3-H), 2.80 (s, 3H, CH3) |
|
21 |
1610 (C=N), 1480, 1440, 1400 (P=N) |
8.05-7.05 (m, 33H, arom), 6.15 (s, 1H, 3-H), 2.40 (s, 3H, CH3) |
|
22 |
3460, 3220 (NH2), 1620 (C=N), 1100, 1070 (tetrazole) |
7.90-7.10 (m, 3H, arom), 6.55 (s, 1H, 3-H), 6.30 (s, 2H, NH2), 2.80 (s, 3H, CH3) |
|
23 |
3460, 3380 (NH2), 1620, 1600 (C=N and C=C) |
7.95-7.10 (m, 3H, arom), 6.40-6.20 (b, 4H, 2 X NH2), 5.85 (s, 1H, 3-H), 2.30 (s, 3H, CH3) |
2-Amino-4-chloro-8-methylquinoline (17)
A similar procedure for hydrolysis of phosphazene 11 was followed for hydrolysis of phosphazene 16 using dilute hydrochloric acid. The acid solution that obtained was neutralized with aqueous sodium hydroxide to give the aminoquinoline 17.
4-Azido-8-methyl-2-(triphenylphosphoryanylideneamino)quinoline (18)
Using the same procedure for obtaining compound 5, compound 18 was prepared from compound 17 and sodium azide in DMF.
2-Amino-4-azido-8-methylquinoline (19)
[a] A similar procedure for hydrolysis of phosphazene 16 was applied to phosphazene 18 to give the aminoquinoline 19.
[b] From compound 17 and sodium azide in DMF, using the same procedure for obtaining compound 5, the compound 19 was prepared.
9-Methyl-5-(triphenylphosphoranylideneamino)[1,2,3,4]tetrazolo[1,5-a]quinoline (20)
A similar procedure to that followed to prepare compound 11 was used starting with azidotetrazoloquinoline 10 and triphenylphosphine in boiling benzene.
2,4-Di(triphenylphosphoranylideneamino)-8-methylquinoline (21)
[a] A mixture of azidotetrazoloquinoline 10 (0.01 mol), triphenyl-phosphine (0.022 mol) and 1,2-dichlorobenzene (50 ml) was heated under reflux for 4 h. Then, the solvent was removed under vacuum and the residue so obtained was crystallized.
[b] Similar to phosphazene 16, compound 21 was afforded from tetrazoloquinoline 20 and triphenylphosphine in 1,2-dichlorobenzene.
[c] Similar to phosphazene 11, compound 21 was also obtained from azidoquinoline 18 and triphenylphosphine in benzene.
5-Amino-9-methyl[1,2,3,4] tetrazolo[1,5-a]quinoline (22)
The phosphazine 20 was treated with hydrochloric acid, following the same method described for hydrolysis of compound 16.
2,4-Diamino-8-methylquinoline (23)
Compound 21 (0.01 mol) was treated with hydrochloric acid (50 ml, 6 M) using the method described for compound 11 to give compound 23.
References and Notes
1. M. Abass, Synth. Comm., 30, 2735 (2000).
2. M. M. Ismail and M. Abass, Acta Chim. Sloven.,(2000) Accepted for publication.
3. M. Abass and M. M. Ismail, Chem. Papers, 51, 186 (2000).
4. H. Staudinger and J. Meyer, Helv. Chim. Acta, 2, 635 (1919).
5. Yu. G. Gololobov, I. N. Zhmurova and L.F. Kasukhin, Tetrahedron, 37, 437 (1981).
6. W. Steinschifter and W. Stadlbauer, J. Prakt. Chem., 336, 311 (1994).
7. P. Vanek, Synth. Comm., 30, 1503 (2000).
8. M. E. Belli, G. Illuminate and G. Marino, Tetrahedron, 19, 345 (1963).
9. G. R. Newkome and W. W. Paudler, Contemporary Heterocyclic Chemistry, Wiley and Sons Inc., New York, (1982).
10. T. Sasaki, K. Kanematsu and M. Murata, Tetrahedron, 28, 2383 (1972).
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0088 as the message subject of your e-mail.