[A0090]
Preparation and Photochemistry of Thiophene-S-oxides
Thies Thiemann,*a Kazuya Arima,b Daisuke Ohira,b Kazuja Kumazoe,b Shuntaro Matakaa
aInstitute of Advanced Material Study
and bGraduate School of Engineering Sciences, Kyushu University, 6-1,
Kasuga-koh-en, Kasuga-shi, Fukuoka 816-8580, Japan
E-mail:
[email protected]
(for TT)
Received: 15 August 2000 / Uploaded: 20 August
Abstract: A number of thiophene-S-oxides were prepared from the corresponding thiophenes. Their reactivity upon photoirradiation was studied.[1] It was found that the photoreactivity of the thiophene-S-oxides depends greatly on their substitution pattern.
Keywords: Thiophene-S-oxides, Photoextrusion, Furan, Deoxygenation
INTRODUCTION
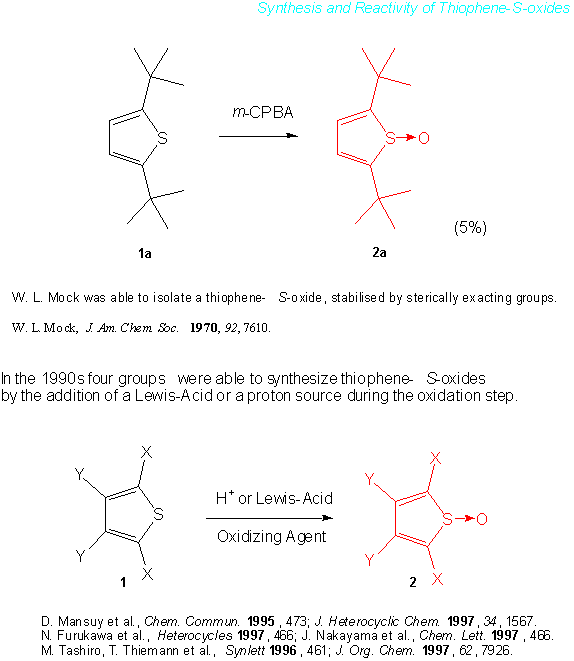
Thiophene-S-oxides, as opposed to the more stable dibenzothiophene-S-oxides,[2] constitute a class of molecules that until fairly recently has been quite elusive. Thus, only in 1970 has the first synthesis and isolation of thiophene-S-oxides been reported, albeit in low yield.[3a] Subsequently, namely in the mid-1990s, thiophene-S-oxides could be accessed more routinely by two main pathways. The first is the direct oxidation of thiophenes with a peracid in the presence of a Lewis acid or a proton acid.[3b-d] In the absence of the acid the major products of the oxidation are the stable thiophene-S,S-dioxides. The acid may serve a number of functions in the oxidation: a.) it may activate the peracid; b.) it may complex to the thiophene-S-oxide formed, thus decreasing the electron density on sulfur and making it less prone to a second oxidation step (to the thiophene-S,S-dioxide). While the oxidations of thiophenes to thiophene-S,S-dioxides are run at rt or at elevated temperatures in the absence of an acid, typically oxidation reactions in their presence (to the thiophene-S-oxides) are run at –20°C. In the second pathway to thiophene-S-oxides substituted zirconacyclopentadienes are reacted with SO2.[4]

The ready availability of the compounds has led to the first studies on the reactivity of thiophene-S-oxides, much of which, however, still remains unknown. Thiophene-S-oxides have been found to be good dienes in Diels-Alder type reactions. Interestingly, non-consumed thiophene-S-oxide often can be reisolated from these reaction mixtures even after protracted periods of time at 60°C. This, however, does not only depend on the substituents on the frame of the thiophene-S-oxide, but also on the nature of the dienophile present in these reactions. For the most part some amount of the corresponding thiophene can also be isolated, most likely formed by deoxygenation of the thiophene-S-oxide. In the presence of certain methylenecyclopropanes, e.g. bicyclopropylidene, an appreciable amount of thiophene-S-oxide reverts to the thiophene, even at temperatures as low as 40°C. A transfer of oxygen from the thiophene-S-oxide to the methylenecyclopropane has not been ascertained to date.
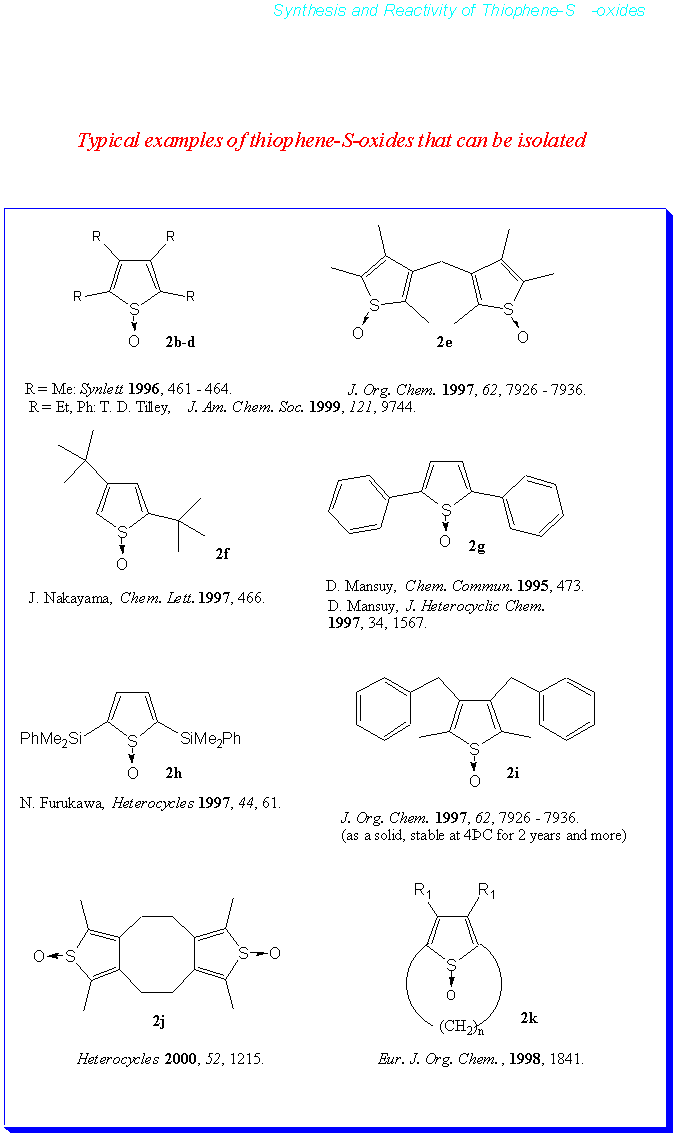
Thiophene-S-oxides have been found to be active against a number of cancer cell strains. Whether this activity is attributable to a slight but continued deoxygenation of the compounds, is still under study. It is clear, however, that compounds such as 2i can be stored in the solid state in the dark and at 0°C for 2.5 years with only an insignificant deterioration of the samples. In solution and upon exposure to light, e.g. daylight, thiophene-S-oxides enjoy a much shorter lifetime and appreciable amounts of thiophenes can be isolated from the ensuing mixtures. To have a more detailed understanding of this process, the authors have started to study the photochemical behavior of thiophene-S-oxides.
RESULTS AND DISCUSSION
The thiophene-S-oxides were prepared by the oxidation of the corresponding thiophenes with meta-chloroperoxybenzoic acid in the presence of BF3.Et2O. All reactions were performed in CH2Cl2 at –20°C. The yield in the preparation of 2,5-bis-(tert.-butyl)thiophene-S-oxide could be improved significantly. All thiophene-S-oxides could be purified by column chromatography on silica gel.
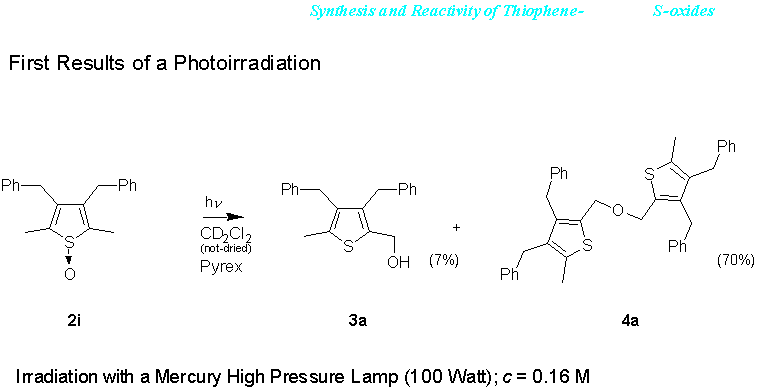
When 3,4-dibenzyl-2,5-dimethylthiophene-S-oxide (2i) was photoirradiated in CD2Cl2 at l > 320 nm a rapid deoxygenation at the sulfur occurred. The two products 3a and 4a could be isolated. Whether hydroxylation at the methyl groups proceeds exclusively via oxygen transfer from sulfur could not yet be ascertained. Two different experiments, one run in completely deaerated and diligently dried CD2Cl2, the other run in completely deaerated but non-dried CD2Cl2 show a slightly different distribution of products. In dried CD2Cl2 also the thiophene can be isolated. A similar behaviour is shown for thiophene-S-oxide 2m in the Scheme. This indicates that hydroxylation of the methyl group does not exclusively proceed by oxygen transfer from sulfur, but that water may be involved in a second, concurrent pathway. This latter pathway may proceed via an addition to 2-methylene-2,5(5H)-dihydrothiophene-S-oxide or 2-methylene-2,3(3H)-dihydrothiophene-S-oxide, possible intermediates formed by 1,3-H (or 1,5-H-)shift. The same pathway may also explain the formation of ether 4a. When ethanol (5 equiv. vs. thiophene-S-oxide) is added to the solution before photolysis, the thienylmethylethylether 4c is the main product that can be isolated.



The photoirradiation of dibromodimethylthiophene-S-oxide 2n leads to a number of products. Here the photoirradiation seems to lead to bromo radicals, thus losing the possibility of a selective conversion of the starting material.
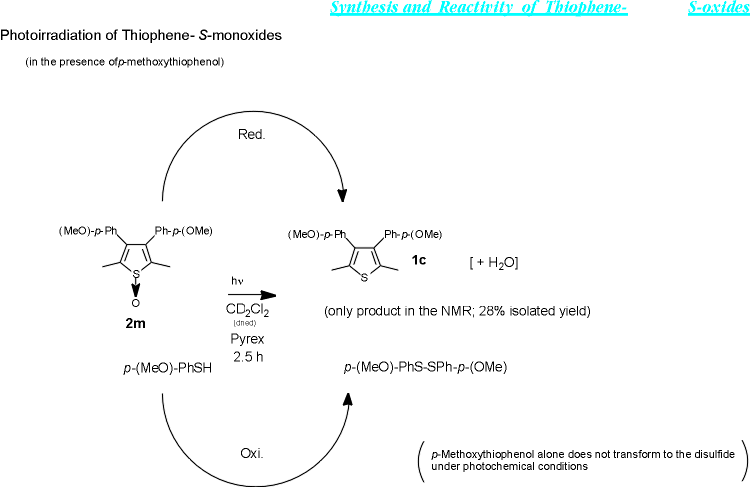
It is not yet certain what species of oxygen is released in the deoxygenation process to yield the thiophenes themselves. While it is possible that an excited thiophene-S-oxide dimer is involved in the process, leading to oxygen molecularly released, the release of an oxenoid species,[6] which reacts with the substrate (oxidation) or the solvent is also conceivable. It must be noted that 2,5-tert-butylthiophene-S-oxide (1a), which may be too hindered to form a dimer, forms a totally different product spectrum. Expectedly in this case no hydroxylation takes place as there is no benzylic position available; however, also very little thiophene is formed in the absence of another oxidizable substance (i.e. in absence of p-methoxythiophenol, see below).
When oxidizable substances such as amines or thiols were added to a solution of 2m in CD2Cl2, photoirradiation under otherwise identical conditions produced the thiophene 1c exclusively. When p-methoxythiophenol was used as an additive in the photoirradiation, bis(p-methoxyphenyl)disulfide, a formal oxidation product of the thiophenol, could be isolated in amounts equal to the thiophene-S-oxide consumed. The reaction does not occur upon photoirradiation of the thiophenol alone and progresses very slowly when the thiophene-S-oxide and the thiophenol are reacted in the dark. The latter result coincides with the slow rate of deoxygenation noted for the thiophene-S-oxides in the dark or under exclusion of direct sun-light.
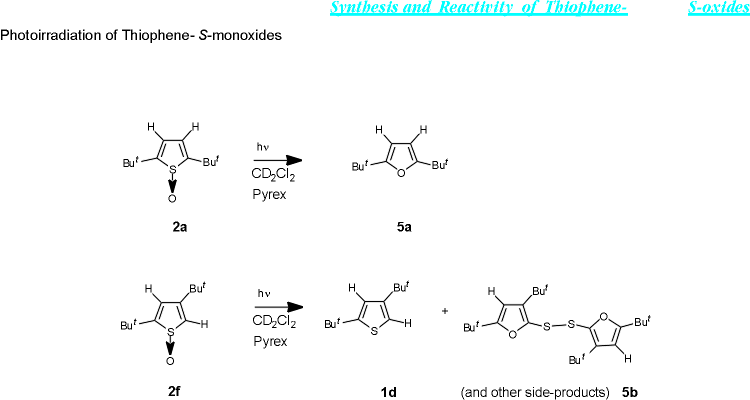
When 2,5-bis(tert-butyl)thiophene-S-oxide (1a), a molecule in which both benzylic positions are quatenary, is photoirradiated a complete conversion to 2,5-bis(tert-butyl)furan (5a) occurs. It is known that 2,5-bis(tert-butyl)furan itself is photoactive at shorter wavelength, but under the reaction conditions used in this communication the compound is quite stable.
When p-methoxythiophenol is added to the solution before photoirradiation, mainly 2,5-bis(tert-butyl)thiophene can be isolated.
2,4-Bis(tert-butyl)thiophene-S-oxide (2f) upon photoirradiation mainly gives 2,4-bis(tert-butyl)thiophene, but several other by-products can also be detected. Of these the most notable is the bis(furyl)disulfide 5b. The mechanism of the photoconversion of the thiophene-S-oxides to the furans 5a and 5b is currently under investigation.
EXPERIMENTAL PROCEDURE
For the irradiation of 2i, 2m and 2n a Rikoh-Kagaku-Sangyo RIKO 100 W high-pressure mercury lamp was used, for the photoirradiation of 2a and 2f a Rikoh-Kagaku-Sangyo RIKO 1kW high-pressure mercury lamp.
The thiophene-S-oxides were prepared according to literature procedures [3,4-dibenzyl-2,5-dimethylthiophene-S-oxide (2i),3c,5a 2,5-dimethyl-3,4-bis(p-methoxyphenyl)-thiophene-S-oxide (2m),5e 3,4-dibromo-2,5-dimethylthiophene-S-oxide (2n)1, 2,4-bis(tert-butyl)-thiophene-S-oxide[7] (2f)].
A representative procedure is as follows: BF3.Et2O (19.3 mL, 153 mmol) was added to 1a (3.0 g, 15.3 mmol) in dry CH2Cl2 (5 mL) at –18°C. Then a solution of m-CPBA (3.4 g, 19.7 mmol) in dry CH2Cl2 (5 mL) was added dropwise to the solution. After the reaction mixture was stirred at –18°C for 3h, it was poured into conc. aq. NaHCO3 and stirred for 30 min. The two phase mixture was extracted with CH2Cl2, washed with water and dried over MgSO4. The organic solvent was removed in vacuo and the residue purified by column chromatography (hexane/ether 1:2) to give 2a (1.53 g, 59%).
Representative spectral data of the compounds [NMR spectra measured at 270 MHz (proton) and 67.8 MHz (carbon) in CDCl3 unless noted otherwise: 3,4-dibromo-2,5-dimethylthiophene-S-oxide (2n): dH 2.33 (s, 6H); dC 13.50, 122.71, 145.34; 2,5-dimethyl-3,4-bis-(p-methoxyphenyl)thiophene-S-oxide (2m): dH 2.26 (s, 6H), 3.78 (s, 6H), 6.77 (d, 4H), 6.84 (d, 4H); dC 11.55, 55.19, 113.53, 125.65, 130.69, 140.90, 141.58, 159.25; 2,5-bis(tert-butyl)thiophene (1a): dH 1.26 (s, 18H), 6.46 (s, 2H); dC 32.53, 34.32, 120.22, 154.05; 2,4-bis(tert-butyl)thiophene (1d): dH 1.27 (s, 9H), 1.37 (s, 9H), 6.71 (s, 1H), 6.77 (s, 1H); dC 31.25, 32.58, 33.47, 34.43, 114.38, 120.50, 152.22, 156.96; 2,5-bis(tert-butyl)thiophene-S-oxide (2a): dH 1.38 (s, 18H), 6.18 (s, 2H); dC 30.24, 35.18, 120.77, 161,51; MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 213 (MH+, 100); 2,4-bis(tert-butyl)thiophene-S-oxide (2f): dH 1.11 (s, 9H), 1.34 (s, 9H), 6.30 (d, 1H), 6.42 (d, 1H); dC 28.28, 30.26, 33.57, 35.31, 122.46, 125.16, 154.81, 166.34; 3,4-dibenzyl-2-hydroxymethyl-5-methylthiophene (3a): dH 1.43 (s, 1H, OH), 2.36 (s, 3H), 3.73 (s, 2H), 3.79 (s, 2H), 4.67 (s, 2H), 6.98 – 7.24 (m, 10H); dC 14.07, 33.03, 33.20, 58.56, 126.38, 126.52, 128.30, 128.48, 128.84, 128.95, 134.75, 136.42, 137.84, 140.34, 140.61; 2-ethoxy-3,4-bis-(p-methoxyphenyl)-5-methylthiophene (4c): dH 1.23 (t, 3H), 2.37 (s, 3H), 3.52 (q, 2H), 3.77 (s, 6H), 4.45 (s, 2H), 6.75 (d, 2H), 6.76 (d, 2H), 6.92 (d, 2H), 6.97 (2H); dC 14.27, 15.27, 55.09 (2C), 65.52, 65.79, 113.12 (2C), 113.26 (2C), 128.57, 128.93, 131.32 (2C), 131.39 (2C), 132.43, 134.23, 138.60, 140.86, 158.00, 158.22; 2-bromomethyl-3,4-dibromo-5-methylthiophene (4d): dH 2.45 (3H), 4.66 (s, 2H); dC 16.20, 26.00, 113.14, 115.46, 131.86, 136.04; 2,5-bis(tert-butyl)furan (5a): dH (CD2Cl2) 1.16 (s, 18H), 5.71 (s, 2H); dC (CD2Cl2) 162.00 (C-2/5); MS (70 eV) m/z (%) 180 (M+, 100); bis-2,2’-(3,5-bis[tert-butyl]furanyl)disulfide (5b): dH 1.13 (s, 18H), 1.27 (s, 18H), 5.90 (d, 2H); dC 28.93, 31.20, 31.32, 32.98, 104.60, 136.71, 144.73, 166.76.
REFERENCES
All comments on this poster should be sent by e-mail to (mailto:[email protected])
[email protected] with A0090 as the message subject of your e-mail.