|
4th International Electronic Conference on
Synthetic Organic Chemistry (ECSOC-4),
www.mdpi.org/ecsoc/,
September 1-30, 2000
|
|
|

Institute of Chemistry, Karl-Franzens-University of Graz
Heinrichstrasse 28, A-8010 Graz (Austria)
E-mail:
Received: 27 August / Uploaded: 27 August 2000 |
| Contents: |
|
1. Introduction
|
| ß-Amino acids and their peptides have most recently found intense interest in the research community [1].Oligomers ("foldamers") of unnatural amino acids have a wide range of potential applications. They may adopt compact, specific conformations. They could be used to form helices, turns and sheets and develop new types of tertiary structures. Certainly short peptides of this type have potential pharmaceutical applications. |
|
2. Structure of ß-peptides
|
|
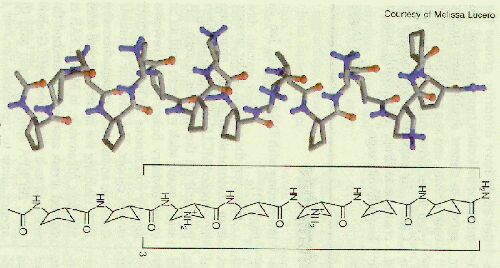
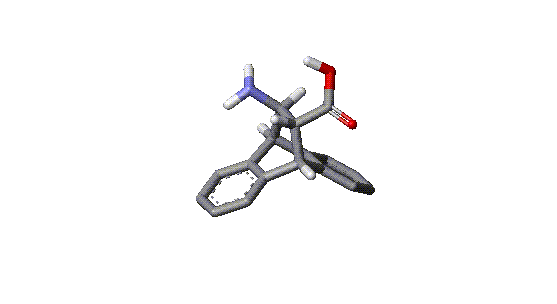
This is a reproduction of ß-17, developed by Sam H. Gellman and coworkers, a ß-peptide active against four species of bacteria, including vancomycin resistant Enterococcus faecium and methicillin resistant Staphylococcus aureus. (red=oxygen, blue=nitrogen and NH). It is composed of the ß-amino acids (R,R)-trans-2-aminopentanecarboxylic acid and (3,R,4,S)-trans-4-aminopyrrolidine-3-carboxylic acid. The peptide folds into a helix similar to that formed by the natural peptide antibiotics called magainins, -that is, it has the hydrophobic side chain on the one side of the helix and the cationic side chain on the other [2]. From the three dimensional structure it is obvious, that a peptide having replaced the aminocyclopentane carboxylate by a similar , but more rigid ß-amino acid type structure could form a helix with interesting properties. We think that two additional condensed aromatic rings as shown in our below depicted target compound could leave enough space to form a helical structure like Gellmanīs ß-17 peptide.
|
|
3. Synthesis
|
|
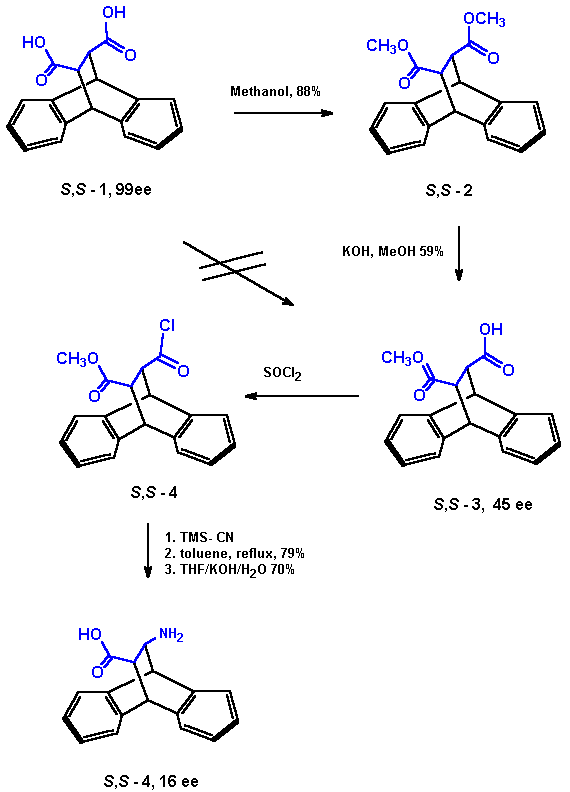
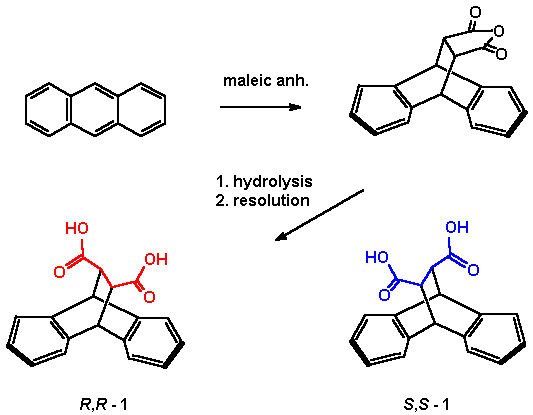 Starting from anthracene and maleic anhydride, Diels Alder cycloaddition , hydrolysis and inversion quantitatively leads to the well known [3] trans -9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboxylic acid 1 (EADC, Scheme 1). This C2-symmetric acid can be easily separated into the enantiomers [3, 6]. 1 has been used previously to prepare the 11,12-diamino analogue, which has found use as catalyst [4] for asymmetric allylic alkylations and as benzoylated variation for an enantioselective synthesis of carbanucleosides [5]. It has also been used by us to prepare a Pirkle-Type chiral stationary phase which especially well separated enantiomers of aryl substituted lactones and cyclic carbamates [6] From racemic 1 we have prepared the racemic title compound in three steps without major problems (Scheme 2). Synthesis of the pure (R,R) and (S,S)-enantiomers proved to be more difficult than expected since partly racemisation occured in the step of alkaline hydrolysis to monoester 3 and also after or during Curtius degradation to the desired amino acid 4. (see Chapter HPLC-analysis below). |
|
|
4. HPLC Analysis of Enantiomers
|
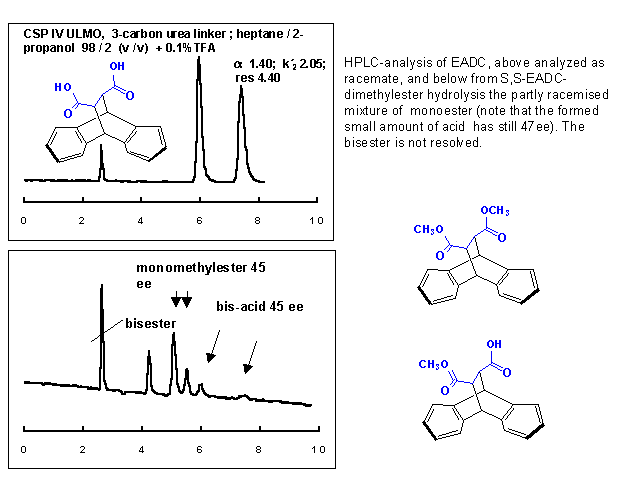
As mentioned above, starting from enantiomer S,S -1, we found significant racemization (45ee) after HPLC analysis of the monomethylester 3.
From previous work we used an optimized chiral stationary phase (CSP) which is based on a ULMO analogue, but contains a short urea linker to silica [6]. Conditions: 25°C; flow 1.0 ml/min; mobile phase n-heptane/2-propanol= 98/2; 0.1% TFA. Under those conditions the separation factor of diacid 1 (resp.monoester 3) was 1.42 (1.17), kī2 2.08 (1.27) and resolution 3.96 (1.90). The enantiomers of the bisester appeared as singlet(kī0.74), but that was well separated from the other four signals.
Despite the loss of enantiomeric purity, it is important that one can easily deduct that the first eluting enantiomer is the (S,S)-monoester, exactly as it has been observed with the acid.
|
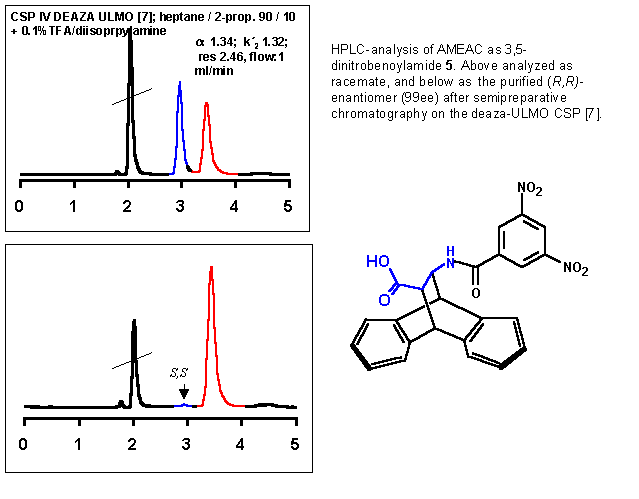
|
|
5. Fmoc- and Boc derivatives
|
|
In order to provide building blocks for peptide synthesis, Fmoc and Boc derivatives of AMEAC were prepared using standard conditions. Both crystalline products could be isolated in good yield and the enantiomers separated on chiral stationary phases. Chiral recognition of the Fmoc-derivative on a commercial preparative p-basic Pirkle naphthyl-CSP (250x20) was sufficient to separate 100 mg of the substance in three runs. 
|
|
6. Conclusion
|
|
We have demonstrated, that Diels-Alder products of anthracene and maleic acid (and fumaric acid derivatives [10]) can be rearranged to give chiral ß-amino acids having a rigid aromatic backbone. The synthesis is streightforward and the yield is acceptable. Separation of enantiomers of AMEAC or the Boc-and Fmoc derivatives is an alternative to the direct synthesis from a enantiopure precursor since partly racemization is there at least a problem which would require careful optimization.
|
|
7. Experimental
|
|
All compounds and solvents were commercially available and used without further purification. HPLC runs were performed at 25 °C and usually monitored at 254 nm. Solvents used for mobile phases were of HPLC grade (MERCK, Darmstadt, Germany)
Instrumentation
|
|
8. Acknowledgement
|
| This paper was designed according to a mask created by Prof. Wolfgang STADLBAUER; some syntheses were performed by Pedro TRAAR and Harald MANG |
|
9. References
|
|
All comments on this poster should be sent by e-mail to (mailto:[email protected]) [email protected] with A0097 as the message subject of your e-mail.