
Figure 1: building of a two-dimensional Dendrimer
[A0098]
Christian Dreyera, Alfred
Blumeb, Monika Bauera,
Jörg Bauera, Jens
Neumann-Rodekircha
aFraunhofer Institute of Reliability and
Microintegration
Branch Lab Polymeric Materials and Composites
Kantstr. 55, D-14513 Teltow, Germany
Phone: +49 3328 46-284 Fax: +49 3328 46-282
Homepage: http://www.epc.izm.fhg.de
E-mail: [email protected]
bMartin-Luther-University Halle-Wittenberg
Institute for Physical Chemistry
Muehlpforte 1, D-06108 Halle, Germany
Phone: +49 345 552-5850 Fax: +49 345 552-7157
Homepage: http://phys.chemie.uni-halle.de/groups/blume/blume_e.html
E-mail: [email protected]
Received: 30 August 2000 / Uploaded: 31 August
1. IntroductionContent1. Introduction
1.1 The Beginning of Dendrimers
1.2 Ways of Synthesis
2. Experimental
2.1 Generation 0
2.1.1 Experimental Example
2.2 Generation 1
2.2.1 Synthesis of G1(C6)-OPh
2.2.2 Synthesis of Generation 1 from G1(C6)-OPh
3. Conclusions
4. Acknowledgements
5. References
Dendrimer is a compound word, composed of the Greek "dendron", meaning tree and the word "polymer", that itself is a fusion of the Greek "poly" (many) and "meros" (part). Dendrimers can be divided in three zones: The region of the initiator core, the region of the inner branching units and the outer level, carrying the endgroups. Figure 1 shows the building of a two dimensional Dendrimer.

Figure 1: building of a two-dimensional Dendrimer
The chemistry of the Dendrimers is a modern and new working field, that brought out a lot of interesting and aesthetic molecules. In future the synthesis of further interesting structures can be expected. The first work [1] initiated a great, almost exponentially, growing number of publications [2]. This new field of research is of academic interest and the industrial research hopes of new positive properties belonging to this new class of molecules, too.
1.1 The Beginning of Dendrimers
Already in 1952 Flory [3], [4] postulated the dendritic branching during polymerisation of monomers of the type A-Bn (n>1). He realized that the linking of the functional groups A and B of these monomers can never lead to a network. The structure resulting from this polymerisation has a great number of branches, but it cannot build a macroscopic network. Figure 2 illustrates the principle of Florys branching polymers.

Figure 2. The Principle of Florys branching Polymers
Because of these statistical reactions, Flory obtained
a broad range of molecular mass distributions, but no well defined single
structures. It was not until 1978 that Voegtle et al [1]
synthesized highly branced cascade-like Oligoamines by repetitive synthesis.
The Dendrimers consist of identical substructures,
like a tree or a fern frond. They remind on the fractals, which were made
public by B. Mandelbrot [5], [6],
[7].
Helge von Koch's Snowflake is another example for a "selfsimilar" structure.
 |
 |
Figure 3. Computer-generated Fractal and Kochs Snowflake.
Because of their building principle the Dendrimers have a great number of endgroups, ideally localized in the outer shell. The space for the endgroups grows with r2, but the volume they need grows exponentially; the result is the occuring of a limit [8]. Above this limit it is not possible to transform the endgroups quantitatively.
There are two principal ways for a repetitive synthesis of Dendrimers :
The divergent synthesis and the convergent synthesis [9], [10], [11], [12].
The divergent route starts with the later core. The core is a molecule with reactive groups X, which react with molecules of the type YZn, where Y is the reactive group and Z is the protected group X. Figure 4 shows the repetitive building of a Dendrimer by the divergent way.

Figure 4. Divergent Synthesis Route.
The convergent way starts with the later outer-sphere of the Dendrimer. In a first step the periphery is synthesized independently from the initiator core by combining the monomers to so-called Dendrons. In the last step the Dendrons were put together to form the Dendrimer.
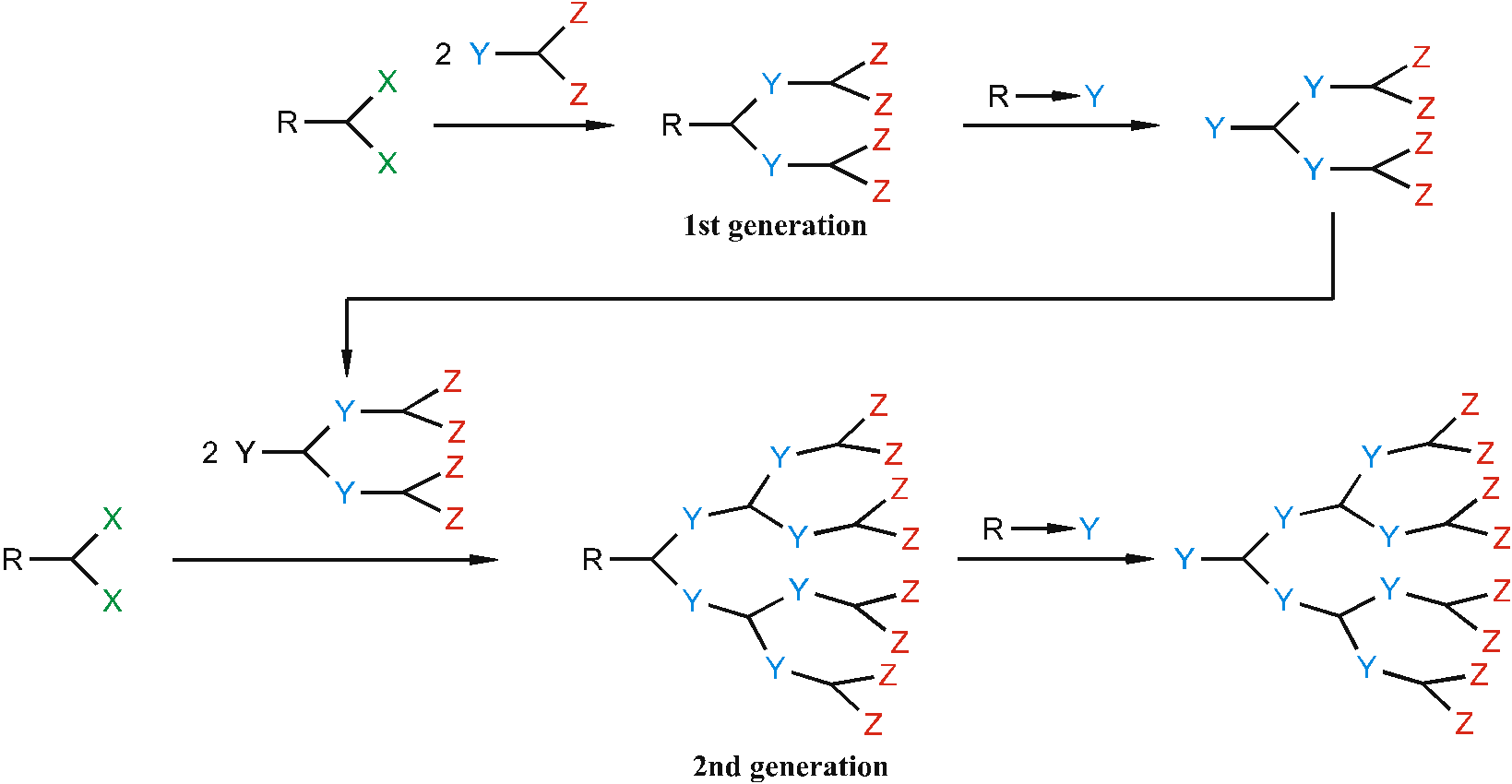
Figure 5. Convergent Synthesis Route
There is a large variety of possible applications for Dendrimers, like highly functional cross-linkers, connecting of catalytical metal fragments, because of their well-defined size as standards for HPLC, GPC and MS. Experiments with DNA showed, that it is possible to bring genes into the cell nucleus [13].
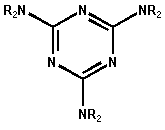
Triazine-based chemistry has been successfully done in our research group for years. Therefore we used the 1,3,5-Triazine-ring (1) as a branching unit. As a spacer we used 1,6-Diaminohexane, which has a favourable length and is commercially available. The outer-sphere contains Dialkyamines with different chain lengths. We examined the incorporation of the obtained Dendrimers in Lipids with DSC and Langmuir-technique, what is reported elsewhere [14].

1
Figure 6. 1,3,5-Triazine ring (1)
For simplification in this electronic paper the following nomenclature is used for the Dendrimers:
Gn(Cm)-R
n is the number of the generation, m the length of the diaminoalkylspacer and R is the group of the outer-sphere.
Figure 7 shows an energy minimized animated 3D-model of the generation 0 Dendrimer with Dioctylamine outer-sphere. The Chime-plugin is needed to view it. Chime can be obtained for free under http://www.mdli.com/support/chime/default.html. Use the mouse with pressed left button to rotate, with shift and left mouse button to zoom. Press the buttons under the picture to change the shape of the Dendrimer.
2,4,6-Trisphenoxy-1,3,5-triazine (TPOT 2), which is commercially available, was used as starting-compound for synthesis of the 0th generation.
The mechanism of the reaction of TPOT (2)
with Dialkylamines is the nucleophilic aromatic substitution [15].The
following mechanism, known as Aminolysis, is propagated for the reaction
:

|
2
|
3
|
|
Figure 8. Aminolysis of TPOT.
As known from the literature [16], the reactivity of TPOT with primary amines is strongly graded for the substitution of the second and the third phenoxygroup. For example Octadecylamine reacts seven times faster with the first phenoxygroup than with the second and 350 times faster than with the third. Secondary amines react even slower [17].
This is confirmed by the experimental results, belonging to the reaction of TPOT with Dibutyl- and Dioctylamine. TPOT and 3.5 mole-equivalents of amine in NMP were heated under reflux in nitrogen atmosphere. Timedepending HPLC-measurements show that the first Phenoxy-group was substituted by the amine after a few hours. The substitutions of the second and third group were very much slower. After eight days of reaction time the part of completely substituted TPOT was less than 5%. Because of this slow reaction TPOT was dissolved in pure amine and the solution was caused to react in an autoclave at 260°C.
Figure 9 shows the different successful syntheses of Generation 0 Triazine-Dendrimers.

|
2
|
5a - e |
Figure 9. Successful Syntheses of Generation 0 Dendrimers.
Synthesis of G0-NOct2: 1.76g (5 mmol) TPOT (2) were dissolved in ca. 15 ml Dihexylamine. The solution was put in a three neck flask and reacted 24h in a heating jacket at 250°C. The HPLC showed almost complete conversion of the TPOT to the desired product (figure 10).
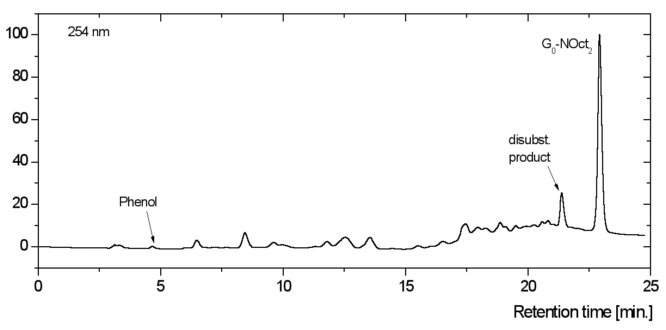
Surplus amine and the built phenol were removed by reduced-pressure distillation. 7.58 g of a dark brown oil were obtained. Corresponding a yield of the crude product of more than 100% because of remaining Dioctylamine. The further purification took place by prep-scale chromatography (THF:Water 75:25). 210 mg of the in THF solved crude product were used. 49 mg of G0-NHex2 were isolated as a yellow thick oil, that solidifies after some hours or by scratching with the spatula.
Structure determination of G0-NOct2:
|
melting point:
elemental analysis: calculated: experimental: |
34°C
C51H102N6 (M = 799,42 g× mol-1) C: 76,63 H: 12,86 N: 10,51 [%] C: 76,91 H: 12,63 N: 09,46 [%] |

5c |
A mass spectrum was recorded to prove the presumed structure. The measured molpeak at 798,1 g/mol matches within the accuracy of the mass spectrometer with the calculated molar mass of 799,4 g/mol.
Further proof of the proposed structure was done
by 1H- and 13C-NMR measurements. Figure 11
shows the 1H-NMR spectrum of G0-NOct2
and the table of the chemical shifts, measured on a Bruker 360MHz machine.
Figure 11. 1H-NMR data of G0-NOct2
Figure 12. Coupled and decoupled 13C-NMR spectra and table of the chemical shifts.
Starting with Trisaminohexylamine (TAHM) (6) the first stage compound G1(C6)-OPh (7) of the generation 1 Dendrimers was obtained by reaction with TPOT (2).
4.23 g (10 mmol) TAHM were dissolved in 100 ml NMP and poured into a solution of 11.07 g (31 mmol) TPOT in 200 ml NMP. The reaction mixture was stirred in a flask under nitrogen for three days at room temperature.
The inspection of the reaction by HPLC and GPC (Figure 13) leads one to suppose that the transformation was successful. Though a side product (10%) was examined. The supposed barbell-like product (Figure 14) was identified later by the MALDI-spectra of the Generation 1 Dendrimer.

After adding 300 ml of 0.5 molar sodium hydroxide solution, a chewing-gum-like mass precipitated. The solid was decanted, in ca. 150 ml dichloromethane dissolved and extracted with water to remove residual NMP. After drying the organic phase over sodium sulphate the solvent was removed by evaporation. 6.75g (5.55 mmol) of a light yellow foam were obtained; this is a yield of 56%. The product was used without further purification.
Figure 13. Reaction of TAHM (6) with TPOT (7) and the resulting products 7 and 8.
2.2.2 Synthesis of Generation 1 from G1(C6)-OPh
Figure 14 shows an energy minimized animated 3D-model of the generation 1 Dendrimer with Dioctylamine in the outer-sphere. The Chime-plugin is needed to view it. Chime can be obtained for free under http://www.mdli.com/support/chime/default.html.Use the mouse with pressed left button to rotate, with shift and left mouse button to zoom. Press the buttons under the picture to change the shape of the Dendrimer.
Figure 14. Energy minimized molecular model of the Dendrimer G1(C6)-NOct2.
The following reaction scheme shows the further successful transformations of G1(C6)-OPh.
Figure 15. Reaction of G1(C6)-OPh
with different Dialkylamines.
Experimental Example
Synthesis of G1(C6)-NBu2: The reaction of Dibutylamine with G1(C6)-OPh was done analogous to the 0th generation 12 hours at 260°C in the autoclave.
1.70 g (1.4 mmol) G1(C6)-OPh (7) were dissolved in 60 ml Dibutylamine. The solution was distilled under reduced pressure and 1.45 g of a dark brown oil were obtained (yield: 73%).
The further purification was done by prep-scale chromatography (THF:water 70:30) analogous to the 0th generation. 130 mg of the crude product were dissolved in 1 ml THF. After evaporation of the pure fractions, drying, dissolving in chloroform, filtration and drying 40 mg of a clear viscous oil were obtained.
Structure determination of G1(C6)-NBu2
|
elemental analysis:
calculated: experimental: |
C78H150N24 (M = 1424,23 g× mol-1) C: 65,78 H: 10,62 N: 23,60 [%] C: 66,28 H: 10,72 N: 20,50 [%] |

9a |
Figure 15 shows a MALDI-spektrum of a sample, containing both supposed products, G1(C6)-NBu2(9a) and the barbell-product, because it wasn't possible to isolate the last one. The MALDI-spektrum contains beside other peaks the molepeak with 1423.5 u, which matches within the accuracy of the mass spectrometer with the calculated molar mass of 1424.23 u of the Dendrimer G1(C6)-NBu2 (9a).
Figure 15. MALDI-TOF of G1(C6)-NBu2 (9a) and barbell-G1(C6)-NBu2.
Another peak with 2384.6 u proves the assumed barbell-product with the molar mass 2385.68 u. The occurrence of further small peaks in the range between 2000 and 3000 u shows the existence of other by-products, whose structure wasn't determined.
Further improvement of the structure was done by 1H-NMR spectroscopy.
Figure 16 shows The 1H-NMR-spektrum
of G1(C6)-NBu2 (9a).
Figure 16. 1H-NMR-spektrum of G1(C6)-NBu2 and table of the data.
Starting with the triazine ring as branching unit,
four different Dendrimers of the 0th generation were synthesized
by reaction of 2,4,6-Trisphenoxy-1,3,5-triazine (TPOT 2) with Dialkylamines
of different chain length at 260-280°C:
|
3 a-d a) R = Butyl; b) R = Hexyl; c) R = Octyl; d) R = Decyl. |
Figure 17. Synthesized Dedrimers of Generation 0.
The reaction of N,N',N''-Tris(6-aminohexyl)melamine (TAHM 6) with three equivalents of TPOT (2) yields to G1(C6)-OPh (7), which reacts with Dialkylamines to the following three Dendrimers of the 1st Generation:
|

7, 9 a-c## 7 R = Phenoxy; 9a) R = Butyl; 9b) R = Hexyl; 9c) R = Octyl |
Figure 18. Synthesized Dedrimers of Generation 1.
The synthesis of larger Dendrimers wasn't successful so far.
For further work it can be mentioned that Dendrimers based on Melamine can be used for various applications. Instead of the alkylchains in the outer-sphere, different functional groups can be used. In this way, a great number of reactive groups on very small space are at disposal. Diethanolamine, for example, instead of Dialkylamines in the outer-sphere will build polyfunctional cross-linkers.
Many thanks to the research group Woerle, Bremen and Dr. M. Dotzauer, Kaiserslautern for NMR-measurements, to Mrs. Wagenknecht, Teltow and Ms. B. Dusch, Kaiserslautern for elemental analysis, Dr. Krueger, Adlershof for MALDI-measurements and to Mr. A. Dreyer for help with the animated gifs.
| E. Buhleier, W. Wehner, F. Vögtle, Synthesis, (1978), 155-158. | |
| Jörg Issberner, Rolf Moors, Fritz Vögtle, Angewandte Chemie, (1994), 106, 2507-2514. | |
| P. J. Flory Principles of Polymer Chemistry, Cornell University Press, Ithaca, New York, 1953. | |
| P. J. Flory, J. Am. Chem. Soc., (1952), 74, 1513+2718. | |
| B. B. Mandelbrot Fractals: Form, Cance and Dimensions, W. H. Freeman, New York, 1977. | |
| B. B. Mandelbrot The Fractal Geometry of Nature, W. H. Freeman, New York, 1982. | |
| B. H. Kaye A Random Walk Through Fractal Dimensions, VCH Verlagsgesellschaft/VCH Publishers, Weinheim/New York, 1989. | |
| Donald A. Tomalia, Adel M. Naylor, William A. Goddard III, Angewandte Chemie, (1990), 102, 119-238. | |
| Holger Frey, Klaus Lorenz, Christian Lach, Chemie in unserer Zeit, (1996), 30, 75-85. | |
| in Advances in Dendritic Macromolecules (Hrsg.: George R. Newkome), Jai Press Ltd., Hampton Hill, 1994, Bd. 1. | |
| J. Gleick Chaos: Making a New Science, Viking Penguin, New York, 1987, S. 99. | |
| Rolf Moors in Dendrimere Neue Kaskadenmoleküle und ihre funktionellen Derivate, Dissertation (Hrsg.: Rheinische Friedrich-Wilhelms-Universität zu Bonn), Bonn, 1994. | |
| Donald A. Tomalia, Spektrum der Wissenschaft, (1995)(September), 42-47. | |
| Christian Dreyer, Diplomarbeit, 1996 | |
| J. March Advanced Organic Chemistry, Reactions, Mechanisms and Structure, 4. Aufl, John Wiley & Sons, New York, 1992. | |
| Jack T. Thurston, James R. Dudley, Donald W. Kaisers, Ingenuin Hechenbleikner, Frederic C. Schaefer, Dagfrid Holm-Hansen, Journal of the American Chemical Society, (1951), 73(7), 2984-2986. | |
| J. Bauer, J. Neumann-Rodekirch, M. Bauer, paper in preparation. |
All comments on this poster should be sent by e-mail to (mailto:[email protected]) [email protected] with A0098 as the message subject of your e-mail.