Figure 1a |
Figure 1b |
[B0002]
Solid Phase Coupling of Benzoic Acid to Wang Resin: A Comparison of Thermal Versus Microwave Heating.
Alexander Stadler and C. Oliver Kappe*
Institute of Chemistry / Organic and Bioorganic Chemistry, Karl-Franzens-University
Graz, A-8010 Graz, Austria.
Fax: ++43-316-3809840; E-mail: [email protected];
URL: http://www-ang.kfunigraz.ac.at/~kappeco
Received: 4 August 2000 / Uploaded: 5 August 2000
Abstract: This presentation reports the possibility to carry out simple solid phase coupling reactions not only by conventional thermal heating but also by microwave dielectric heating. Thus coupling of benzoic acid to Wang resin occurs under microwave irradiation within 3 h in excellent yield (>99%) as compared to 72 h at room temperature. However, a further increase of reaction rates in this particular coupling reaction is not possible due to a side reaction that occurs at higher temperatures.
Introduction
Combinatorial chemistry has revolutionized drug discovery and many other
branches of chemistry and related fields. Today there is an ever growing demand for large
libraries of compounds that are being evaluated for their biological and other properties
using high throughput screening (HTS) protocols. One of the cornerstones of combinatorial
chemistry has been the development of solid-phase organic synthesis (SPOS) based on the
original Merrifield method for peptide synthesis. Since reactions on polymer supports
enable chemical reactions to be driven to completion, and allow simple purification of
products, combinatorial chemistry has been primarily carried out by SPOS. However,
solid-phase synthesis still exhibits several shortcomings due to the nature of
heterogeneous reaction conditions. Nonlinear kinetic behavior, slow reactions, solvation
problems, and degradation of the polymer support due to the long reaction times are some
of the problems experienced in SPOS. Only very recently the first reports have emerged
which demonstrate that microwave activation can be employed to speed up solid-phase
reactions significantly [1] (for further details on microwave-enhanced chemistry, click here).
In this context we have carried out a thorough investigation of several microwave-enhanced
solid-phase organic reactions. In contrast to earlier published experiments [1] we have
used commercial microwave reactors from Milestone
MLS (Ethos 1600 series), that allow temperature and pressure control inside the
reaction vessel. This instrument features a built-in magnetic stirrer, direct temperature
control of the reaction mixture with the aid of a shielded thermocouple, and software that
enables online temperature/pressure control by regulation of microwave power output. The
microwave reactor can either be fitted with standard glassware and a reflux condenser for
operation at atmospheric pressure (Figure 1a), or can be equipped with a sealed PFA vessel
for carrying out reactions at elevated pressure (Figure 1b).
Figure 1a |
Figure 1b |
Figure 1. ETHOS 1600 Microwave reactor with normal pressure (1a) and sealed vessel (1b) setup.
Results and Discussion
One of the most common solid-phase reactions in combinatorial synthesis
involves the coupling of an acid (i.e. an amino acid) to a Wang-type resin using
diisopropylcarbodiimide (DIC) as coupling reagent [2]. The current work employs the
conventional carbodiimide method for coupling aryl carboxylic acids to polystyrene resins
[3]. As a solid support we have used standard polystyrene Wang resin (1.0 equiv. OH/g, 1 %
DVB cross linked). The resin is swollen in the solvent mixture DCM/DMF 9:1 (10 ml) and
treated with excess (3 equiv.) of DIC. Benzoic acid is added (5 equiv.) and after addition
of a catalytic amount of DMAP the reaction mixture is being stirred under a variety
of thermal/microwave conditions for several hours to permit complete coupling of the acid
to the resin (see below).
In all experiments the loading of the resin is estimated from FTIR measurements of the
resin (KBr pellet) and also determined accurately by cleavage of the loaded resin with
trifluoroacetic acid (TFA). Thus, the loaded polymer is treated with about 1 ml of
the "cleavage cocktail" (TFA/DCM 1:1) per 100 mg of resin. The mixture is
stirred for 30 min at room temperature and subsequently filtered by suction. After removal
of the solvent pure benzoic acid was regenerated. The remaining resin is now carrying the
trifluoroacetyl residue [4].
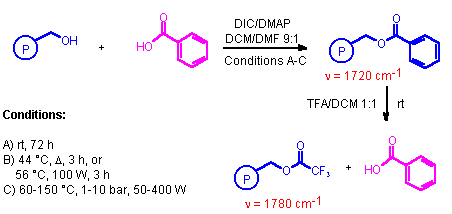
Scheme 1
For comparison purposes this coupling reaction was carried out under a set of different conditions, involving a) standard coupling conditions at room temperature; b) thermal heating or microwave superheating at reflux temperature at atmospheric pressure (44 �C and 56 �C, respectively); and c) microwave superheating under sealed vessel conditions (50-400 W, 1-10 bar, 60-150 �C).
A) Ester Coupling at Room Temperature:
The coupling reaction was conveniently monitored by FTIR-measurements as demonstrated in Figure 2, which shows the increasing intensity of the ester carbonyl absorption at 1720 cm-1 going from 10 min to 180 min reaction time. By subsequent cleavage the exact yields were determined for reaction times of 18 h (66%), 48 h (87%), and 72 h (99%). Complete conversion for this model reaction at room temperature (25 �C) therefore requires ca. 3 days.

Figure 2. FTIR Monitoring of Benzoic Acid Coupling to Wang Resin.
B) Ester Coupling at Reflux Temperature (Thermal Versus Microwave Heating):
As one would expect the speed of this coupling process is significantly
enhanced by performing the solid phase coupling at reflux temperature, i.e. 44 �C
for the DCM/DMF 9:1 mixture. Here, a complete loading of the resin was achievable within 3
h (Figure 3).
Superheating of solvents at atmospheric pressure is a common phenomenon when dealing with
microwave heating of polar organic solvents [5]. The origin of this superheating effect
has been rationalized in terms of an "inverted heat transfer" effect (from the
irradiated medium towards the exterior) preventing the onset of nucleate boiling [5]. For
the particular DCM/DMF 9:1 mixture (including reagents) the reflux temperature at 100 W
was determined to be 56 �C (setup as shown in Figure 1a). As can be seen in Figure 3, no
further improvement of yield can be achieved by switching from conventional to microwave
heating. On the contrary, it appears that early on in the experiment the conversion rates
are lower in the microwave run than under thermal conditions. At the present time no
explanation for this phenomenon can be given.
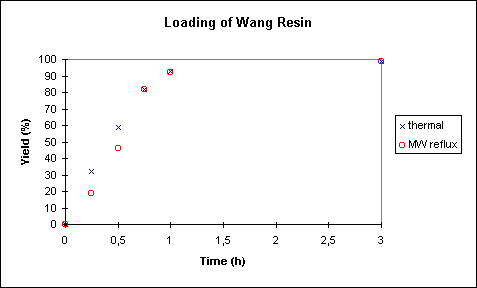
Figure 3. Loading of Wang Resin under Thermal (44 �C) and Microwave (56 �C) Conditions (1 atm)
C) Ester Coupling under Microwave Superheating and Sealed Vessel Conditions:
Since we wanted to further decrease the reaction time by microwave
activation we decided to repeat the above microwave experiments in a closed vessel system
under pressure. For this purpose the reaction mixture was placed inside the setup shown in
Figure 1b and initially irradiated at 100 W. This method leads to coupling in 73% yield
after 15 min, which unfortunately seemed to be the maximum achievable conversion. Further
increase of the reaction time diminished the yield to 59 % after 30 min and 44% after 60
min. Similarly, an increase in microwave input power (200 W, 400 W) resulting in higher
temperatures and pressure also had a negative effect on the loading (Table 1). While it is
tempting to assume that at higher temperatures some cleavage of the material from the
resin has occurred, independent studies have confirmed that the resin used is quite stable
in its free (OH) and loaded form (O2CPh), and no cleavage from the loaded resin
occurs under resubjection to the reaction conditions.
MW-Power [W] |
Time (min) |
T [�C] |
Pressure [bar] |
Loading (%) |
50 |
15 |
68 |
1.5 |
48 |
100 |
15 |
89 |
2.7 |
73 |
200 |
15 |
132 |
8.8 |
53 |
400 |
15 |
153 |
10.1 |
50 |
The reason for this unsatisfactory result is most probably the rearrangement of the DIC-activated acid (i.e. the isourea) under the reaction conditions to the thermodynamically more stable N-acylurea (Scheme 2) [6]. Apparently, at higher temperatures, such isourea derivatives preferably undergo rearrangement rather then coupling to the corresponding polymer bound benzylic alcohol.
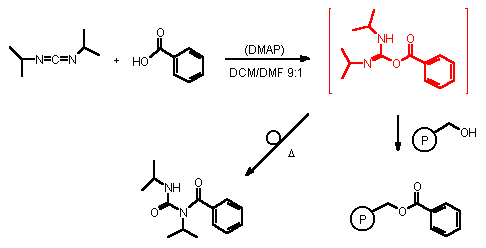
Scheme 2
In subsequent studies we have found that the formation of the N-acylurea
does not require the presence of DMAP. Even in the absence of DMAP the formation of this
product can be observed as shown by independent studies. It is formed in 58% yield within
60 min at 100 �C (3 bar, 100 W), having a mp of 114 �C.
We have found however, that by modifying the solvent mixture for the coupling reaction
this unwanted side reaction can be suppressed and a decent loading of the resin with
benzoic acid can after all be achieved. Pure DCM as a solvent leads to 84% yield after
irradiation for 3 h under pressure, a mixture of DCM/THF 9:1 even provides 95% yield after
only 1 h of irradiation under pressure (Table 2).
Time (h) |
CONV |
MW I |
MW II |
MW III |
0.25 |
32 % |
19 % |
29 % |
53 % |
0.50 |
59 % |
46 % |
57 % |
77 % |
0.75 |
82 % |
82 % |
68 % |
89 % |
1.00 |
93 % |
92 % |
73 % |
95 % |
3.00 |
99 % |
99 % |
84% |
CONV: DCM/DMF 9:1, thermal reflux, 44 �C.
MW I: DCM/DMF 9:1, microwave superheating, 56 �C, 1 atm.
MW II: DCM, microwave superheating, 89 �C, 3 bar.
MW III: DCM/THF 9:1, microwave superheating, 89 �C, 3 bar.
Conclusions
In summation it is shown that microwave irradiation does reduce the time required for
complete loading of Wang resin with benzoic acid as compared to room temperature
conditions. Coupling of benzoic acid to Wang resin at thermal reflux leads to identical
conversion rates and yields as microwave induced reflux at atmospheric pressure. At higher
temperatures the thermodynamically favored rearrangement of the isourea intermediate
becomes the preferred reaction pathway, therefore yields are decreasing significantly.
These results clearly demonstrate that care must be taken in the application and
interpretation of microwave assisted solid phase chemistry.
Acknowledgments
This work was supported by the Austrian Science Fund (FWF, Project P-11994-CHE).
References and Notes
[1] See, for example: Yu, H. M.; Chen, S. T.; Wang, K. T. J. Org. Chem., 1992, 57, 4781; Larhed, M.; Lindeberg, G.; Hallberg, A. Tetrahedron Lett., 1996, 37, 8219; Hoel A. M. L.; Nielsen, J. Tetrahedron Lett., 1999, 40, 3941.
[2] Barry A. Bunin, "The Combinatorial Index", Academic Press, 1998.
[3] Ley, S.; Myngett, D. M.; Koot, W., Synlett, 1995, 1017.
[4] Florencio Zaragoza Dorwald, "Organic Synthesis on Solid Phase", Wiley-VCH, 2000, page 33.
[5] Baghurst, D. R.; Mingos, D. M. P. J. Chem. Soc. Chem. Commun., 1992, 674; Saillard, R.; Poux, M.; Berlan, J.; Audhuy-Peaudecerf, M. Tetrahedron, 1995, 51, 4033.
[6] Mathias, L. S., Synthesis 1979, 561.
All comments on this poster should be sent by e-mail to (mailto:[email protected]) [email protected] with B0002 as the message subject of your e-mail.