[B0004]
Nitrobenzenesulfonylated Amide Linkers on Solid Support: Useful Starting Materials for Diversity Generation
Eduard R. Felder* and Andreas L. Marzinzik
Novartis Pharma AG - Core Technology Area - CH-4002 Basel, Switzerland.
Current address: Eduard Felder Pharmacia&Upjohn, Discovery Research Oncology, Via Pasteur 10, I-20014 Nerviano, Italy
E-mail:
Received: 9 August 2000 / Uploaded: 15 August
Introduction
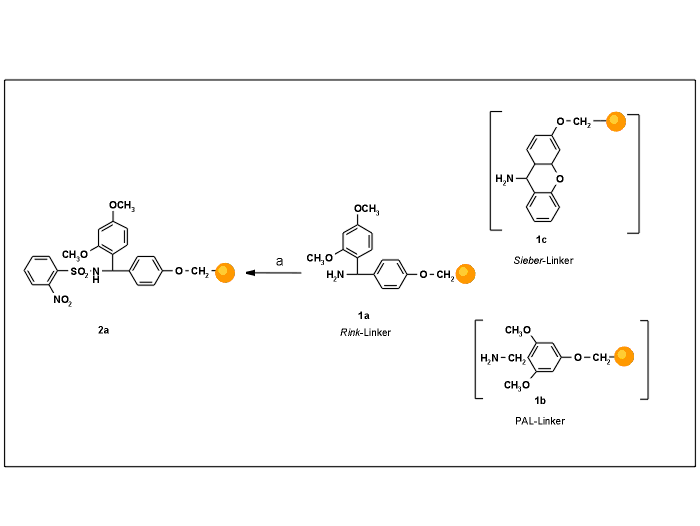
The Rink-linker (1a) [1] and analogous linkers like 1b [2] and 1c [3] are commonly used not only in peptide chemistry, but also in combinatorial chemistry, for grafting components with a carboxylic group onto the solid phase. Once assembled, the synthesis products are released into solution as carboxamides by acid treatment.
When we first proposed the o-nitrobenzenesulfonylation of such linkers [4], adapting the chemistry described by Fukuyama et al. [5] in solution, we emphasized the usefulness of these derivatives for enabling a straightforward introduction of diversity at the site of attachment to the linker. After deprotection, the amino functionality is maintained (as a secondary rather than primary amine, see 3a-c) and is available for many of the same diversification schemes already validated in the past. At the time, it was more common to prepare primary carboxamides, without considering the exploitation of the additional diversity accessible with variably N-substituted, analogous secondary amides. Meanwhile the basic strategy of introducing diversity with a branch point on the linker function has become more widespread. In most reported examples the solid phase starting material carries an acid labile linker with an aldehyde function, ready to be converted to secondary amines by reductive amination with amines [6,7]. Conversely, the classical amide linkers known from peptide chemistry, may be derivatized to the same type of secondary amine resins with aldehyde solutions, thus inverting the choice of solid phase grafted reactant [8].
Results and Discussion
Here, we illustrate the simplicity of our proposed method [4], using transiently o-nitrobenzenesulfonylated amide linkers for introducing diversity with building blocks of the alcohol type, i.e. with reagents readily available from a large commercial choice.
The o-nitrobenzenesulfonyl-derivative 2a is a useful starting material for diverging into a large variety of established combinatorial synthesis schemes introducing diversity at the site of attachment to the linker. The arylsulfonylamide function is acidic enough to enable alkylation by Mitsunobu reaction with primary, and to a more limited extent even with secondary alcohols. After cleavage of the o-nitrobenzenesulfonyl moiety, the resulting secondary amines are ready for further derivatization (see Scheme 3). Usage of secondary alcohols in the alkylation step leads to derivatives, which are not accessible by reductive alkylations, thus avoiding the obligatory presence of the methylene group formed during the reduction.

Scheme 1. Activation of amino-resins for subsequent Mitsunobu reaction.
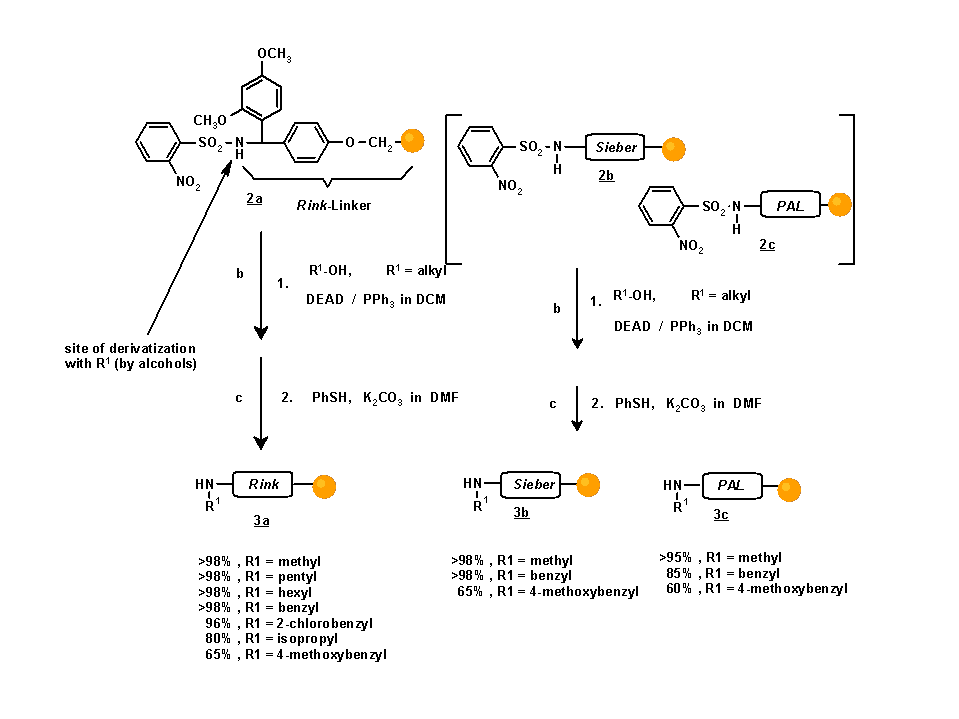
Scheme 2. Preparation of a variety of (mono-)alkylated resins for subsequent diversification
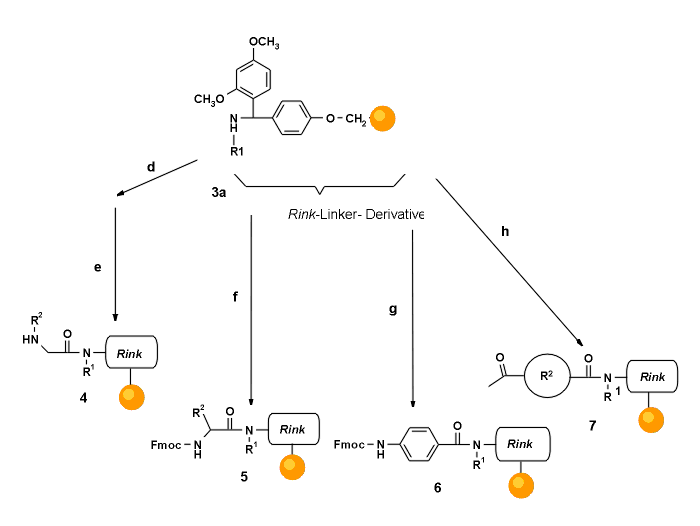
Scheme 3. Acylation of secondary amino-resins.
For each derivatization, the amount equivalent to 50
mMol commercially available, polystyrene based amino-resin (Rink Amide resin, Sieber Amide resin, PAL resin) was used (approx. 100 mg). Yields were determined by cleavage of resin aliquots and integration of the HPLC UV detection trace (peak assignment by electrospray MS and comparison with known references). Where possible, cleavage of Fmoc protection groups with 20% piperidine in DMA and UV spectrophotometry at 299 nm was used as well.The reaction conditions are summarized in Table 1.
Table 1.
|
a |
o-NO2-C6H4-SO2Cl (11 eq.), DMAP (1 eq.) in DCM / pyridine 5/1, 4 h |
|
b |
R1-OH (10 eq.), DEAD (10 eq.), PPh3 (10 eq.) in DCM, 2 x 2 h |
|
c |
Thiophenol (12 eq.), K2CO3 (27 eq.) in DMF, 2 x 2 h |
|
d |
Br-CH2-COOH (10 eq.), DIC (12.5 eq.) in DMA, 2 x 30 min |
|
e |
2.5 M R2-NH2 (40 eq.) in DMSO, 2.5 h |
|
f |
Fmoc-aa (6 eq.), HATU (6 eq.), DIPEA (12 eq.) in DMA, 2 x 4 h |
|
g |
Fmoc-NH-4-C6H4-COOH (3 eq.), tetramethyl- a-chloroenamine [9] (3 eq.), DIPEA (5 eq.) |
|
h |
Acetyl-R2-COOH (3 eq.), HATU (3 eq.), DIPEA (6 eq.) in DMA, 2 x 4 h |
The observed yields are summarized in Fig. 1
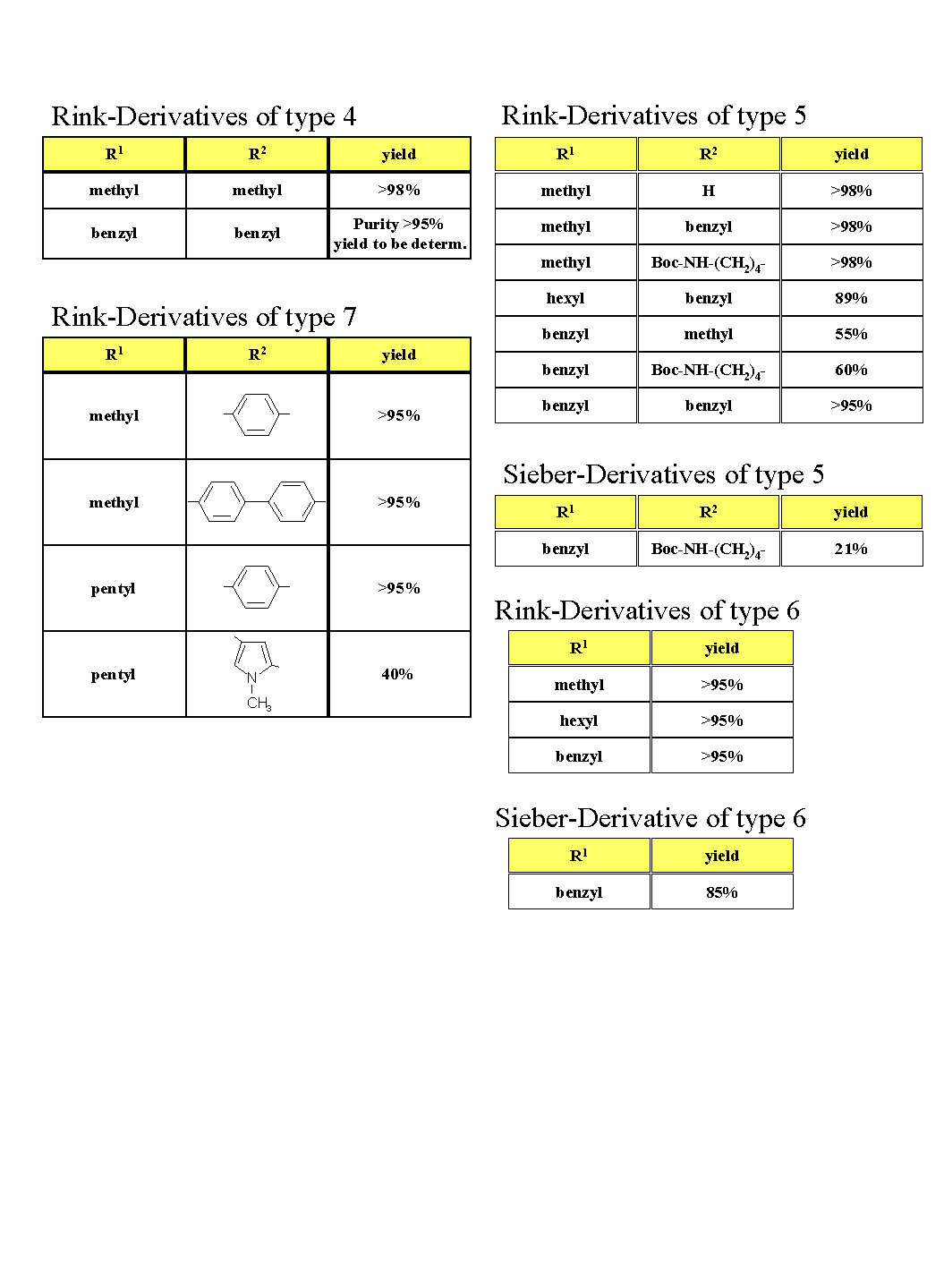
Figure 1
Conclusions
N-alkylated Rink-linkers are useful intermediates for the solid phase preparation of combinatorial libraries. The described method is a straightforward approach for branched diversification at the linker attachment site, the primary amino group of commercial amide linkers. The building blocks used for generating diversity are primary alcohols and (to a more limited extent, depending on the steric hindrance involved) also secondary alcohols.
A variety of linker types are in principle amenable to this approach. Of the three linkers studied in our work, the Rink-linker turned out to be superior in the acylation steps following its derivatization. Nitroarylsulfonylated Rink-linker is therefore proposed as a versatile starting material for this methodology. Room for improvement is expected from less hindered linkers. With bulky substituents, the resulting secondary amino group remains difficult to acylate.
References
[1] Rink, H., Tetrahedron Lett. 1987, 28, 3787-3790.
[2] Sieber, P., Tetrahedron Lett. 1987, 28, 2107-2110.
[3] Albericio, F.; Barany, G., Int. J. Pept. Prot. Res. 1987, 30, 206-211.
[4] Felder, E.; Marzinzik, A., poster presented at the 3rd Annual High-Throughput Organic Synthesis Symposium (Cambridge Healthtech Institute) 1998, San Diego.
[5] Fukuyama, T.; Jow, C.-K.; Cheung, M., Tetrahedron Lett. 1995, 36, 6373-6376.
[6] Fivush, A.M.; Willson, T.M., Tetrahedron Lett. 1997, 38, 7151-7154.
[7] Sarantakis, D.; Bicksler, J.J., Tetrahedron Lett. 1997, 38, 7325-7328.
[8] Chan, W.C.; Mellor, S.L., J. Chem. Soc. Chem. Commun. 1995, 1475-1477.
[9] Munyemana, F. et al., Tetrahedron Lett. 1989, 30, 3077-3080.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with B0004 as the message subject of your e-mail.