Scope and Limitation of a Tin Promoted Amidation on Solid Phase: A New Monitoring for the T1 Triazene Linker [1]

[B0007]
|
Scope and Limitation of a Tin Promoted Amidation on Solid Phase: A New Monitoring for the T1 Triazene Linker [1] |
|
RWTH Aachen, Institut für Organische Chemie, Professor-Pirlet-Str. 1, D-52074 Aachen, Germany
E-mail: [email protected]
Received: 11 August 2000 / Uploaded: 23 August
The amidation of a benzoic acid derivative attached to solid support via the T1 triazene linkage has been described. In the presence of a tin amide reagent, smooth amidations take place with primary and secondary amines to give benzamides. Anilines failed under the reaction condition employed. The progress and the success of the reaction were monitored by 1H NMR spectroscopy using a novel direct cleaving method.
The solid phase synthesis of organic compounds has gained a renaissance for the last years to due the fact, that parallel reactions necessary for high-throughput synthesis can efficiently be used operating with resin chemistry [
3]. Among the various linkers applied for attachment and detachment of organic fragments [4] multifunctional cleavage, [4e] i. e. functionalization by detachment, promised additional diversity, which would not bias the libraries produced. Especially the triazene linker for arene, called T1 linker, has been described to show the versatility of diazonium type anchoring and its suitability for multifunctional detachment [5]. Although cleavage methods are investigated to a larger extent, modifications on the bead to show the scope and limitation of this linker are somewhat still lacking.The synthesis of amide bonds is (due to its relevance in natural product and drug like synthesis) a very important tool in solid phase synthesis. Various methods exist for the coupling of carboxylic acids to amines (peptide coupling procedures). The presence of a free carboxylic acid function might be problematic when using the acid sensitive T1 linker. The conversion of esters to amides might be more practical in the sense of stability of intermediates. The direct amidation has been conducted with the amine component as the solvent at 70-100 °C [
6a,b], a process that is suitable for simple amines. Lithium or sodium bases have been used to facilitate the carbon nitrogen bond formation [6c,d]. The most promising method was reported by Roskamp et al., who used a bis(bis(trimethylsilyl)amino)tin (II), Sn[N(SiMe3)2]2, for the smooth conversion of esters to amides [7a]. The yields reported by Roskamp et al. encouraged us to use this reagent on solid support.Thus, starting from ethyl p-aminobenzoate (1), diazotation and coupling to the amine resin 2 [
8] resulted in the formation of the triazene resin 3.
Initial experiments with piperidine as the nucleophile in the amidation reaction revealed, that an excess of the amine and Sn[N(SiMe3)2]2 was crucial for the success of this reaction. Besides this, a temperature of 80 °C in toluene was required to force this reaction to completion. After this optimization, we used various amines for this reaction on the Bohdan Miniblock [
9]. While primary and secondary amines such as 4a-f are nucleophilic enough for this coupling, anilines such as p-bromoaniline or p-chloroaniline failed completely.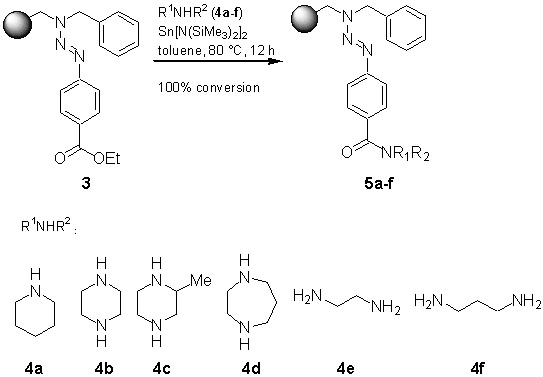
Since the triazene T1 linker could act as a multifunctional anchor [5g], various types of benzamides are anticipated.
Possibilities of the T1 linker system
[5g]
In-situ Cleavage and Monitoring
Monitoring of reaction on solid support is always much more difficult than in liquid phase. During the experiments above, we also encountered this problem. Initially, the success of the reaction was determined using elemental analysis, since the amide formation increases the nitrogen content substantially. As a rule of thumb, one nitrogen atom increases the amount of nitrogen in the elemental analysis by one percent if a resin with a loading of 0.7 mmol/g is used. This method requires only a small amount of material (3-5 mg), however, on-line analytics is difficult to achieve and the accuracy of the results is not very exact. Similarly, these drawbacks are present for IR spectroscopy although for the amidation reactions the IR stretchings of the starting material (strong, 1700 cm-1) change visibly upon product formation (strong, 1640-1625 cm-1). Thus, we developed a system, which allows a rapid monitoring of reactions conducted with the T1 linker.
A small amount (50-100 mg) of resin [
3] was placed in a glass pipette containing a plug of glass wool. A mixture of [D]-trifluoroacetic acid in [D4]-methanol (10% v/v) was used to cleave the diazonium salt directly into a NMR tube at room temperature.The use of [D]-trifluoroacetic acid instead of TFA was not crucial, however, in 1H spectra with prolonged recording times (e. g. 2D experiments) a shift of the signal corresponding to the acidic proton was observed. This cleavage reaction was used for various T1 resins and so far, both electron deficient and electron rich diazonium salts are suitable, however, some diazonium ions decompose within 24 hours. For the amidation reaction, the progress of the transformation could be followed easily because of the characteristic signal of the ester group [6.45 ppm (quart, 2 H, J = 7.14 Hz), 1.46 ppm ( t, 3 H, J = 7.14 Hz).
It is interesting to note, that the solvent used in the last washing step was incorporated in nearly all cases to a measurable extent into the resin, regardless of the nature of the solvent and the drying time/pressure.
Conclusion
The Roskamp reagent for conversion of esters to amides was successfully employed for the T1 linker. While secondary and primary amines react smoothly to give the desired products, anilines failed so far. The reaction progress was monitored using a new in-situ cleaving method utilizing standard 1H NMR spectroscopy.
This work was supported by the Deutsche Forschungsgemeinschaft (BR 1750/2-1) and the Fonds der Chemischen Industrie (Liebig stipend to S. B.). We thank the companies NovaBioChem, Bayer AG, and Grünenthal GmbH for donations of chemicals and financial support, Bohdan Inc. for the opportunity to use the MiniBlock synthesizer, Prof. Dieter Enders for continuous interest and support of our work as well as Johannes Köbberling for beneficial discussions and contributions concerning this work.
General: The polymeric compounds were characterized by IR and elemental analysis; the products obtained after cleavage were characterized by NMR methods (1H NMR, partially 13C NMR) and MS. The purity was controlled by NMR, HPLC, LC-MS, GC and/or GC-MS. Merrifield resin (1-2% cross-linked, 0.63 mmol g-1, 200-400 mesh) and benzyl amine resin was obtained from CalBioChem-NovaBioChem. All resins were washed sequentially by using a vacuum reservoir connected to a sintered glass frit.
[
1] Part 10 in the series: Nitrogen-based Linkers. For part 8, see: S. Dahmen, S. Bräse, Angew. Chem., in press. For part 7, see Ref. [5d].[2] Undergraduate research student, October 1999.
[3] a) E. M. Gordon, R. W. Barrett, W. J. Dower, S. P. Fodor, M. A. Gallop, J. Med. Chem. 1994, 37, 1385
-1401; b) L. A. Thompson, J. A. Ellman, Chem. Rev. 1996, 96, 555-600; c) J. S. Früchtel, G. Jung, Angew. Chem. 1996, 108, 19-46; Angew. Chem. Int. Ed. 1996, 35, 17-42; d) F. Balkenhohl, C. von dem Bussche-Hünnefeld, A. Lansky, C. Zechel, Angew. Chem. 1996, 108, 2437-2487; Angew. Chem. Int. Ed. 1996, 35, 2288-2337; e) P. H. H. Hermkens, H. C. J. Ottenheijm, D. Rees, Tetrahedron1997, 53, 5643-5678; f) Combinatorial Peptide and Nonpeptide Libraries (Ed. G. Jung), VCH, Weinheim, 1996; g) Combinatorial Chemistry (Eds.: S. R. Wilson, A. W. Czarnik), Wiley-Interscience, New York, 1997, cit. lit.; h) B. A. Bunin, The Combinatorial Index, Academic Press, San Diego 1998, cit. lit..[4] For reviews about linker strategies: a) B. J. Backes, J. A. Ellman, Curr. Opin. Chem. Biol. 1997, 1, 86
-93; b) I. W. James, Tetrahedron 1999, 55, 4855-4946; c) F. Z. Dörwald, Organic Synthesis on Solid Phase: Supports, Linkers, Reactions, John Wiley & Sons, Weinheim 2000; d) F. Guillier, D. Orain, M. Bradley, Chem. Rev. 2000, 100, 2091-2157; e) D. Obrecht, J. M. Villalgordo, Solid-Supported Combinatorial and Parallel Synthesis of Small-Molecular-Weight Compound Libraries, Elsevier, Oxford, 1998, p. 98.[5] a) S. Bräse, D. Enders, J. Köbberling, F. Avemaria, Angew.Chem. Int. Ed. 1998, 37, 3413-3415; Angew. Chem. 1998, 110, 3614; b) S. Bräse, S. Dahmen, J. Heuts, Tetrahedron Lett. 1999, 40, 6201-6203; c) A. de Meijere, H. Nüske, M. Es-Sayed, T. Labahn, M. Schroen, S. Bräse, Angew. Chem. Int. Ed. 1999, 38, 3669-3672; Angew. Chem. 1999,111, 3881; d) M. Lormann, S. Dahmen, S. Bräse, Tetrahedron Lett. 2000, 41, 3813-3816; e) S. Bräse, M. Lormann, Chem. Eur. J. 2000, in preparation; f) for accounts/reviews: S. Bräse, S. Dahmen, M. Lormann, Proceedings of ECSOC-3, The Third International Electronic Conference on Synthetic Organic Chemistry, http://www.mdpi.org/ecsoc-3.htm, The T1 Linker: Multidirectional Cleavage for Solid Phase Organic Synthesis 1999; g) S. Bräse, S. Dahmen, Chem. Eur. J. 2000, 6, 1899-1905.
[6] a) T. T. Sakai, N. Rama Krishna, Bioorg. Med. Chem. 1999, 7, 1559-1566; b) G. Kretzschmar, Tetrahedron 1998, 54, 3765-3780; c) C. Stammel, R. Fröhlich, C. Wolff, H. Wenck, A. de Meijere, J. Mattay, Eur. J. Org. Chem. 1999, 1709–1718; d) A. J. Belfield, G. R. Brown, A. J. Foubister, P. D. Ratcliffe, Tetrahedron 1999, 55, 13285 - 13300.
[7] a)W.-B. Wang, E. Roskamp, J. Org. Chem. 1992, 57, 6101-6103; G. M. Strunz, H. Finlay, Tetrahedron 1994, 50, 11113-11122.
[8] The T1 linker system is commercially available (Calbiochem-Novabiochem, Catalogue 2000). http://www.nova.ch. See also innovation sheet 2/99.
[9] http://www.bohdan.com/miniblock.htm
All comments on this poster should be sent by e-mail to (mailto:[email protected])
[email protected] with B0007 as the message subject of your e-mail.