
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[C0001]
'TaClo', IN VIVO
ANALYTIC, DISPOSITION AND METABOLISM OF A CHLORAL-DERIVED MAMMALIAN ALKALOID WITH NEUROTOXIC PROPERTIESG. Bringmann,1* R. Bruckner,1 M. Munchbach,1 D. Feineis,1 C. Grote,2 W. Wesemann,2 S. Diem,3 M. Herderich3
1 Institute of Organic Chemistry, University of Wurzburg, Am Hubland, D-97074 Wurzburg, Germany.
E-mail: [email protected], [email protected]
2 Institute of Physiological Chemistry, University of Marburg, Hans-Meerwein-Str., D-35033 Marburg, Germany.
3 Institute of Food Science, University of Wurzburg, Am
Hubland, D-97074 Wurzburg, Germany.
Received: 26 July 2000 / Uploaded: 29 July 2000
INTRODUCTION
According to the toxin hypothesis, reactive chemical compounds (e.g. alkanes, carbolines, cyanides, isoquinolines, heavy metals, pesticides) arising from environmental pollution or drug abuse are discussed to be causative factors for the induction of neurodegenerative processes, e.g. Parkinson's disease.
We report on the highly halogenated heterocycle 1-trichloromethyl-1,2,3,4-tetrahydro-b-carboline ("TaClo", 4) that readily originates in vitro under physiological conditions (pH 7.4, T = 37�C) via a Pictet-Spengler type condensation from the biogenic amine tryptamine ("Ta", 1) and the synthetic aldehyde chloral ("Clo", 2) (see Figure 1).[1-3] Its spontaneous formation in humans has generally to be taken into account after application of the hypnotic chloral hydrate or after exposition to the widely used industrial solvent trichloroethylene ("tri", 3), which is known to be metabolized to 2. Furthermore, tri-addiction turned out to severely affect the health of young sniffers.

Figure 1.
Possible in vivo formation of 'TaClo' (4) from the endogenously occurring amine tryptamine ('Ta', 1) and synthetic aldehyde chloral ('Clo', 3) by a Pictet-Spengler type cyclization.Due to the structural analogy of the b-carboline framework of TaClo (4) to the well known parkinsonism-inducing neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP, 5) [4] and due to the fact that 4 contains a highly lipophilic and possibly radical inducing CCl3-moiety similar to the insecticide DDT (6), we postulated 4 to exhibit distinct toxic properties as given in Figure 2:

Figure 2.
The TaClo concept.
Thus, neuropharmacological investigations on 4 seemed rewarding, since TaClo (4)
might be a potential 'natural' inducer of parkinsonian-type neurodegenerative processes.
NEUROTOXICITY OF TaClo
Interestingly, TaClo (4) was indeed found to penetrate the blood-brain barrier[5] and to develop toxic potencies against dopaminergic[6] and serotonergic[7] neurons. From animal studies, it became evident that a 7-weekly subchronic treatment of rats with 4 slowed down the locomotoric activities of the animals and led to an increased sensitivity towards the dopamine agonist apomorphine 9 months after the last application.[8] An acute systemic administration of 4 to rats was further shown to alter nigrostriatal dopamine metabolism[9] and to enhance extracellular serotonin release[10] at more than 300 %.
The pronounced in vivo toxicity of TaClo (4) is supported by its high inhibition capacity towards complex I and II of the mitochondrial respiratory chain.[11] In vitro studies using dopaminergic (TH-IR, IMR-32, HEK-392) and serotonergic (JAR) cell lines revealed TaClo (2) to penetrate cell membranes readily by passive diffusion and not by a selective uptake mechanism, e.g., via neurotransporters. Dose-dependently, 4 significantly inhibited dopamine or serotonin uptake, an effect that strongly correlates with the loss of cell viability.[6,7] For an overview concerning the neurotoxic properties of TaClo (4) known so far see Figure 3.
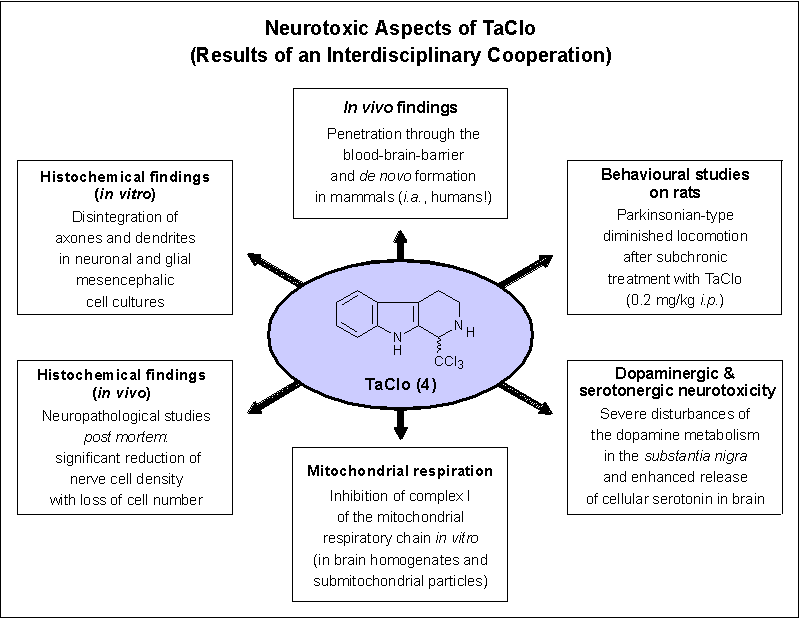
Figure 3.
Neurotoxic properties of the mammalian alkaloid TaClo.
IN VIVO FORMATION OF TaClo IN MAN
Since chloral hydrate does not shorten the REM- and the non-REM-type sleep, it is even nowadays administered as a soporific on a gram scale to elder people as well as to children. Due to the high chemical reactivity of the aldehyde chloral (3) we started to investigate more closely the presumable occurrence of TaClo (4) in humans formed from endogenously present tryptamine (1) and therapeutically administered 3.
The first unambiguous identification of TaClo (4) in blood samples of elderly patients who had been treated with chloral hydrate was achieved by a specific and sensitive gas chromatographic (GC) screening procedure based upon electron-capture (ECD) and mass-selective detection (MSD) after converting TaClo (4) into its volatile trifluoroacetyl derivative. The identity of 4 in humans was clearly demonstrated by GC-MS analysis in selected ion-monitoring mode (SIM), and by the characteristic chlorine isotopic pattern of the molecular ion.[12]

Figure 4.
First identification of TaClo (4) in human blood.
Starting with a second series of investigations on chloral-treated patients, we preferably used modern LC-MS/MS techniques for the identification of TaClo (4) in human blood. This high sensitive method allows us to examine the blood samples directly without any further derivatization step necessary after extraction using RP-18 cartridges. Besides monitoring TaClo (4) by the typical chlorine isotopic pattern of the molecular ion, it is also possible to detect 4 by single reaction monitoring (SRM) experiments. Using this highly selective SRM mode, we are able to detect characteristic fragment ions of TaClo (4), formed by retro-Diels-Alder reaction of the b-carbolines' pyridol ring - neutral loss of 29 amu (CH2NH) - (see Figure 5) and dehydrohalogenation - neutral loss of 36 amu (HCl).[13]

Figure 5.
Retro-Diels-Alder fragmentation and typical chlorine isotopic pattern of the [M+H]+ molecular ion and characteristic SRM chromatogram of TaClo (4) and the internal standard [D4]-TaClo in a blood sample.
Using this relatively smooth but highly selective method we could clearly detect 4
in blood samples of 14 elderly patients and of a ten year old boy after treatment with
chloral hydrate. Further LC-MS/MS investigations on human faeces, liquor, and urine
samples are in progress.
FORMATION OF TaClo FROM TRICHLOROETHYLENE
In order to prove our second hypothesis that the neurotoxic heterocycle TaClo (4) might arise in the course of trichloroethylene (3) metabolism, rat liver microsomes were incubated for 90 min at 37�C in the presence of NADPH with tryptamine (1) and 3. LC-MS/MS analysis clearly revealed TaClo (4) to be formed. From this point of view, the in vivo formation of TaClo (4) in industrial workers or 'tri'-sniffers has seriously to be taken into account.
From an inquiry of 80 Parkinson patients which have been exposed to trichloroethylene (3) over a long periode, first hints were found that a chronic contact with 3 may lead to an early onset of first Parkinsons' symptoms (< 50 a). Concerning the widespread use of 3, further studies have to aim on the neuro-degenerative potency of 3 and the possible involvement of its neurotoxic metabolite TaClo (4).
DISPOSITION AND METABOLISM OF TaClo IN THE RAT ORGANISM
The observed neurotoxic behavior of TaClo (4) in vivo and its de novo formation in humans after chloral hydrate treatment prompted us to synthesize radiolabeled [3-14C]-1-trichloromethyl-1,2,3,4-tetrahydro-b-carboline ([14C]-4).[13] In order to elucidate its pharmacokinetic disposition in the mammalian organism a dose of 0.6 mCi/kg [14C]-4 was applied intraperitoneally to the rat. The radioactivity was measured in organ tissues and body fluids of these treated animals in time intervalls between 0.5 h and 48 h (see Figure 6).

Figure 6. Distribution of radioactivity after application of [14C]-TaClo to the rat.
The radioactivity was rapidly distributed all over the organism and only slowly excreted within 48 h to 35% by renal and to 65% by intestinal elimination. Within the first 6 h, highest metabolic activity was found in kidneys, liver and the small intestine, while there were only low values of radioactivity to be observed in brain, spleen, heart and muscle tissues. Fatty tissue, neighboured to the kidneys and being located in the peritoneal cavity was able to store radioactivity for at least 24 up to 48 h. Especially the high values in liver and the small intestine, and the preferred intestinal excretion give evidence for a great hepatic first-pass effect, which might be finally responsible for the low activity in systemic blood circulation and also in brain tissue. By separating the blood samples, low activity values were detected in the erythrocytes, and only traces in plasma. Although less than 0.1 % of the applied activity was found to penetrate the blood-brain barrier, it has to be mentioned, that this level is degradated slowly within a half-life time of 15.6 h. Thus, an accumulation of endogenously formed TaClo (4) resulting from a long-term treatment of patients with chloral hydrate, or maybe after a long-term exposure to trichloroethylene (3), has seriously to be taken into account.
With the powerful help of tandem mass spectrometry (LC-MS/MS), we succeeded in identifying the main metabolites of 4 in urine and brain samples of rats intraperitoneally treated with 12 mg/kg TaClo (4) (see Figure 7).[13] The structures of these metabolites have been elucidated by mass scan and SRM-experiments as described above, and by comparison of the retention times of synthetically prepared reference compounds. As the main metabolic pathway of TaClo (4), monohydroxylation of its isocycle resulting in the formation of 5-, 6-, 7- and 8-hydroxy-TaClo (7 a-d) has to be stated. These regioisomers were observed to occur in a ratio of 1:8:35:2. Further glucuronidation of 7 a-d leading to the secondary metabolites 5-, 6-, 7- and 8-O-TaClo-glucuronides 8 a-d (ratio: 4:12:35:1), which could be enzymatically cleaved by incubating the urine with glucuronidase. The lipophilic trichloromethyl group is subject of two transformations: hydrodehalogenation generates 1-dichloromethyl-THBC (9), while the complete dechlorination forms the halogen-free heterocycle tryptoline-1-carboxylic acid ('TaGly', 10). Interestingly, 10 is further hydroxylated, but mainly in position 6 of the isocycle, leading to 6-hydroxy-TaGly ('SerGly', 10). With the b-carboline norharman (12) we found the only fully aromatic TaClo metabolite known so far.
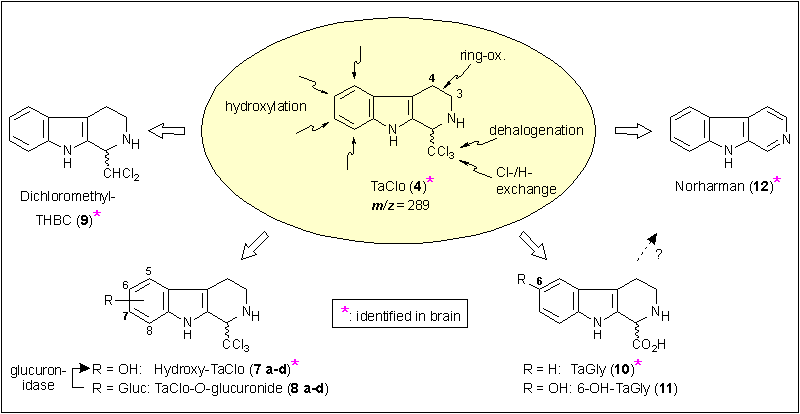
Figure 7.
TaClo (4) and its metabolites in urine and brain samples detected by LC-MS/MS analyses.
For proving the halogen-free metabolites 10, 11, and 12 to be authentic TaClo metabolites and not only constituents of the normal rat catabolism, one animal received 12 mg of 3,3,4,4-tetradeutero-TaClo ([D4]-4). Expectedly, in the urine sample of this animal, [D4]-TaGly ([D4]-10), [D4]-SerGly ([D4]-11) and [D2]-norharman ([D2]-12) were clearly identified.
Furthermore, LC-MS/MS analyses gave no hints neither on dehydrodehalogenation nor on N-
respectively O-methylation products nor on glycine or sulfate conjugates.
REFERENCES
[1] G. Bringmann, R. God, D. Feineis, W. Wesemann, P. Riederer, W.-D. Rausch, H. Reichmann, and K.-H. Sontag, The TaClo concept: 1-trichloromethyl-1,2,3,4-tetrahydro-b-carboline (TaClo), a new toxin for dopaminergic neurons. J. Neural Transm. [Suppl.] 46:231 (1995).
[2] G. Bringmann, D. Feineis, R. God, S. Fahr, W. Wesemann, H.-W. Clement, C. Grote, W. Kolasiewicz, K.-H. Sontag, C. Heim, T.A. Sontag, H. Reichmann, B. Janetzky, W.-D. Rausch, M. Abdel-Mohsen, E. Koutsilieri, M.E. Gotz, W. Gsell, B. Zielke, and P. Riederer, Neurotoxic effects on the dopaminergic system induced by TaClo (1-trichloromethyl-1,2,3,4-tetrahydro-b-carboline), a potential mammalian alkaloid: in vivo and in vitro studies. Biogenic Amines 12:83 (1996).
[3] G. Bringmann, D. Feineis, C. Grote, H.-W. Clement, K.-H. Sontag, B. Janetzky, H. Reichmann, W.-D. Rausch, P. Riederer, and W. Wesemann, Highly halogenated tetrahydro-b-carbolines as a new class of dopaminergic neurotoxins, in: Pharmacology of Endogenous Neurotoxins, A. Moser, ed., Birkhauser, Boston, Basel, Berlin (1998).
[4] M.A. Collins, E.J. Neafsey, B.Y. Cheng, K. Hurley-Gius, N.A. Ung-Chhun, D.A. Pronger, M.A. Christensen, and D. Hurley-Gius, Endogenous analogs of N-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine: Indoleamine derived tetrahydro-b-carbolines as potential causative factors in Parkinson's Disease. Adv. Neurol. 45:79 (1986).
[5] G. Bringmann, H. Friedrich, G. Birner, M. Koob, K.-H- Sontag, C. Heim, W. Kolasiewicz, S. Fahr, M. Stablein, R. God, and D. Feineis, Determination of the dopaminergic neurotoxin 1-trichloromethyl-1,2,3,4-tetrahydro-b-carboline (TaClo) in biological samples using gas chromatography with selected ion monitoring. J. Chromatogr. B 687:337 (1996).
[6] W.-D. Rausch, M. abdel-mohsen, E. Koutsilieri, W. Chan, and G. Bringmann, Studies of the potentially endogenous toxin TaClo 1-trichloromethyl-1,2,3,4-tetrahydro-b-carboline in neuronal and glial cell cultures. J. Neural Transm. [Suppl.] 46:255 (1995).
[7] G. Bringmann, R. Bruckner, R. Mossner, D. Feineis, A. Heils, and K.P. Lesch, Effect of 1-trichloromethyl-1,2,3,4-tetrahydro-b-carboline TaClo on human serotonergic cells. Neurochem. Res. 25:837 (2000).
[8] C. Heim and K.-H. Sontag, The halogenated tetrahydro-b-carboline "TaClo": a progressively-acting neurotoxin. J. Neural Transm. [Suppl.] 50:107 (1997).
[9] C. Grote, H.-W. Clement, W. Wesemann, G. Bringmann, D. Feineis, P. Riederer, and K.-H. Sontag, Biochemical lesions of the nigrostriatal system by TaClo (1-trichloromethyl-1,2,3,4-tetrahydro-b-carboline) and derivatives. J. Neural Transm. [Suppl.] 46:275 (1995).
[10] M. Gerlach, A.-Y. Xiao, C. Heim, J. Lan, R. God, D. Feineis, G. Bringmann, and K.-H. Sontag, 1-Trichloromethyl-1,2,3,4-tetrahydro-b-carboline increases extracellular serotonin and stimulates hydroxyl radical production in rats. Neurosci. Lett. 257:17 (1998).
[11] K.-H. Sontag, C. Heim, T.A. Sontag, W. Kolasiewicz, W. Clement, C. Grote, W. Wesemann, B. Janetzky, H. Reichmann, D. Feineis, R. God, G. Bringmann, D. Rausch, M. Abdel-mohsen, M. Abdel-moneim, W. Chan, B. Zielke, M. Gotz, W. Gsell, and P. Riederer, Progressive neurodegeneration of the dopaminergic system and inhibition of the complex I induced by the chloral-derived tetrahydro-b-carboline TaClo. The Basal Ganglia V (Eds.: C. Ohye, M. Kimura, J.S. McKenzie), Plenum Press New York and London, 387 (1996).
[12] G. Bringmann, R. God, S. Fahr, D. Feineis, K. Fornadi, and F. Fornadi, Identification of the dopaminergic neurotoxin 1-tri-chloromethyl-1,2,3,4-tetrahydro-b-carboline in human blood after intake of the hypnotic chloral hydrate. Anal. Biochem. 270:167 (1999).
[13] G. Bringmann, R. Bruckner, M. Munchbach, D. Feineis, R. God, W.
Wesemann, C. Grote, M. Herderich, S. Diem, K.-P. Lesch, R. Mossner, and A. Storch: Neurotoxic
Factors in Parkinson's Disease and Related Disorders (Eds.: A. Storch, M.A. Collins),
Kluwer Press / Plenum Publishing Corporation, New York (2000).
Please feel free to visit the homepage of Prof. Bringmanns' group at:
www-organik.chemie.uni-wuerzburg.de
For questions during the poster session please contact:
Miriam Munchbach or Doris Feineis
Thank you for your interest!
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with C0001 as the message subject of your e-mail.