
|
|
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[C0004]
 |
|
||
| . | . |
|
Baeyer-Villiger reaction of Wieland-Misher ketone analogs |
|
Unité de Phytopharmacie et Médiateurs Chimiques, INRA, Route de Saint-Cyr F-78026 Versailles Cedex, France Fax 01 30 83 31 19 E-mail [email protected] Received: 26 July 2000 / Uploaded: 9 August |
Introduction :
The role of plant secondary metabolites in host plant recognition, especially in oligophagous insects, is of great importance. Substances eliciting feeding responses of the insects have been extensively studied and numerous attempts have also been made in the past decades to isolate antifeedants from plants avoided by insect species. As the most studied of these plant secondary metabolites with antifeedant activity, but also in some cases insecticide activity, one should cite polygodial 1 1, waburganal 2 2, azadirachtine3 , toosendanine 4 4, clerodanes 5 (clerodine 5) and ecdysteroids 6 (20-hydroxyecdysone 6). However, the diversity of structure of these products, the diversity of their biological activities and their structural complexity did not allow establishment of unambiguous structure-antifeedant activity relationships.
On the other hand, studies have allowed the emergence of a family of sesquiterpenic compounds of the Eudesmane family with significant antifeedant activity: 7 the agarofurans 7 8(figure 1). In the Celastraceae family, numerous polyhydroxylated agarofuran esters have been isolated and studied on account of their potential cytotoxic9, antitumor10 and immunosuppressive11 activities and have been proved to be in some cases insecticides. 7,8 Even if the antifeedant activity of these compounds was in most cases reported as lower than those of the other known compounds cited above, the structural diversity of the agarofurans esters encountered as secondary metabolites should allow in this family of natural products the establishment of structure-activity relationships. For this purpose, it is important to design versatile and efficient strategy toward the synthesis of the tricyclic core of agarofurans.
Albeit several syntheses of agarofurans have been reported in the literature, 12,13 the main problem of these syntheses remains the introduction of the oxygenated heterocycle with the right configurations of the asymmetric centers. Indeed, it forces either the hydroxy-isopropyl substituent on cycle B of the agarofuran to be introduced in an axial position or the configuration at C-6 to be controlled in the last steps of the synthesis devoted to the heterocycle ring closure. 14 We already have published a new synthetic approach toward the synthesis of highly hydroxylated agarofurans15.
Beside this previous work on the synthsesis of
hydroxylated agarofurans, we have developped another approach to these
compounds based on the possible rearrangement of the caprolactones obtained
from the Baeyer-Villiger reaction of Wieland-Misher ketone 16
derivatives 17.
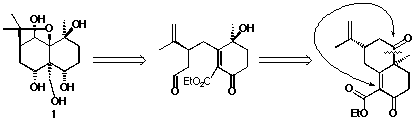
Results :
References : follow this link
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with C0004 as the message subject of your e-mail.