
|
|
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[C0005]
 |
|
||
| . | . |
|
Stereoselective construction of the tetrahydrofuran ring. |
|
Unité de Phytopharmacie et Médiateurs Chimiques, INRA, Route de Saint-Cyr F-78026 Versailles Cedex, France Fax 01 30 83 31 19 E-mail [email protected] Received: 26 July 2000 / Uploaded: 9 August |
The role of plant secondary metabolites in host plant recognition, especially in oligophagous insects, is of great importance. Substances eliciting feeding responses of the insects have been extensively studied and numerous attempts have also been made in the past decades to isolate antifeedants from plants avoided by insect species. As the most studied of these plant secondary metabolites with antifeedant activity, but also in some cases insecticide activity, one should cite polygodial 1 1, waburganal 2 2, azadirachtine3 , toosendanine 4 4, clerodanes 5 (clerodine 5) and ecdysteroids 6 (20-hydroxyecdysone 6). However, the diversity of structure of these products, the diversity of their biological activities and their structural complexity did not allow establishment of unambiguous structure-antifeedant activity relationships.
On the other hand, studies have allowed the emergence of a family of sesquiterpenic compounds of the Eudesmane family with significant antifeedant activity: 7 the agarofurans 7 8(figure 1). In the Celastraceae family, numerous polyhydroxylated agarofuran esters have been isolated and studied on account of their potential cytotoxic9, antitumor10 and immunosuppressive11 activities and have been proved to be in some cases insecticides. 7,8 Even if the antifeedant activity of these compounds was in most cases reported as lower than those of the other known compounds cited above, the structural diversity of the agarofurans esters encountered as secondary metabolites should allow in this family of natural products the establishment of structure-activity relationships. For this purpose, it is important to design versatile and efficient strategy toward the synthesis of the tricyclic core of agarofurans.
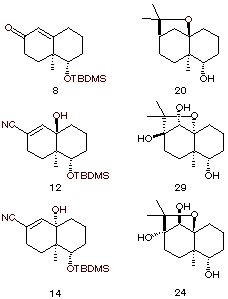
Albeit several syntheses of agarofurans have been reported in the literature, 12,13 the main problem of these syntheses remains the introduction of the oxygenated heterocycle with the right configurations of the asymmetric centers. Indeed, it forces either the hydroxy-isopropyl substituent on cycle B of the agarofuran to be introduced in an axial position or the configuration at C-6 to be controlled in the last steps of the synthesis devoted to the heterocycle ring closure. 14 We already have published a new synthetic approach toward the synthesis of highly hydroxylated agarofurans15(Scheme 1a). However, our previously reported work did not deal with the problem of the introduction of the missing substituent on ring B. In connection with this work, we thus decided to first investigate the stereochemical features related to this problem and to the acido catalysed heterocycle ring closure in model studies starting from easily available hexahydronaphtalenones.
We thus have developed a new strategy of construction of the tetrahydrofuran ring system starting from Wieland-Misher ketone derivative 816(figure 1) and using the substitution of a leaving group at C-617 by a cyanide as a key step (Scheme 1b). In this strategy, the heterocycle ring closure would be effected through the intramolecular trapping by an hydroxy group of a carbocation generated upon acidic treatment either at C-4a (Scheme 1b, route a) or C-10 (Scheme 1b, route b). In the first case, the configuration at C-6 should induce the configuration at C-4a and should thus be fixed previously in the synthesis while configuration at C-4a would have no influence on the stereochemical course of the ring closure reaction. The second case (route b) should force us either to fix the configuration at both C?4a and C?6 before the ring closure process or to only fix the configuration at C-4a and to induce, during the ring closure reaction, a reversible epimerization at C-6; the driving force of such an equilibrium would be therefore the formation of the heterocycle as reported in other related syntheses of the agarofuran skeleton14a.
Results :
|
 |
References : follow this link
Selected experimental data : 12, 14, 17, 24
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with C0005 as the message subject of your e-mail.