
|
|
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[C0007]
 |
|
||
| . | . |
|
|
|
Unité de Phytopharmacie et Médiateurs Chimiques, INRA, Route de Saint-Cyr F-78026 Versailles Cedex, France Fax 01 30 83 31 19 E-mail [email protected] Received: 26 July 2000 / Uploaded: 9 August |
Introduction :
Clerodanes diterpenes have
been considerably studied by biologists and chemists, according to their
interesting antifeedant activity on insects as well as their unique structure,
which make them a challenging target for total synthesis. Several problems
related to their synthesis have been solved1,2,3,
and some natural products have been synthesised in the ajugarin's series4
as well as in the dehydroclerodanes 4c.
However, the problem of the stereoselective construction of the acyclic
C-9-C-115 double bond, drastically related
to this of the stereochemical control of stereogenic centers C-8, C-10
and C-5 remains up to date.
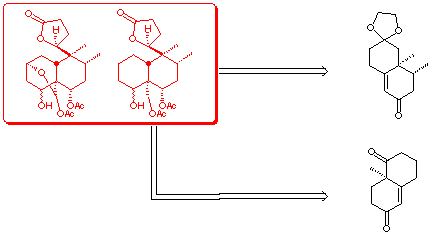
We have developped two stategies aimed to this goal. Both strategies
consist in controlling the configurations of some of the stereogenic
centers of the target molecule in an early bicyclic structure easy accessible.
One of the ring of these precursors should prefigurate the B ring of the
clerodanes, the cleavage of the other ring giving access to functionnalized
side chains which should allow the further elaboration of either the decalinic
moeity of the molecule or the furofuran system through well known methodologies.
 |
|
Route B allows a rapid control of the configurations at C-9 and C-11
but needs a further elaboration of ring B of the clerodin, and especially
the introduction of a methyl group at C-8 and the construction of ring
A. Results obtained have already been published (Ducrot, P.-H.; Hervier,
A.-C.; Lallemand, J.-Y. Synth. Comm. 1996, 26, 4447-4457).
This poster describes results obtained in route A, where the configurations
at C-8, C-9 and C-10 are easily controlled.
Results :
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with C0007 as the message subject of your e-mail.