
|
|
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[C0003]
 |
|
||
| . | . |
|
|
|
Ducrot, P.-H.* Unité de Phytopharmacie et Médiateurs Chimiques, INRA, Route de Saint-Cyr F-78026 Versailles Cedex, France Fax 01 30 83 31 19 E-mail [email protected] Received: 26 July 2000 / Uploaded: 9 August |
Introduction :
In our on-going programs aimed to the discovery of new pesticides of
plant origin, we have extensively studied the composition in secondary
metabolites of various indigenous plants from the Sudan, known for their
promising biological activity and sometimes even used in traditional agriculture
for pest management purposes.
One of our extracts, obtained from Cyphostemma crotalarioides[1]
exhibited a very promising antifungal activity against Fusarium
nivale. In a previous report [2], we have
described the isolation and the identification of resveratrol and some
of its known natural oligomers from the roots of this plant. This type
of compounds was known to be produced by numerous plant species [3]
and proved to exhibit cancer chemopreventive, antifungal and antibacterial
activities [4].
The air-dried, ground roots were macerated with methanol. This extract
showed antifungal activity. The dried extract was then dissolved in water
and partitioned against ethyl acetate. The last fraction was chromatographed
on a reversed phase preparative column packed with silinised silica gel
(Kieselgel). For elution, a chromatography gradient started with H2O 70%
/ MeOH 30% and ended with MeOH 100%, was used. Fifteen fractions were collected.
Each biologically active fraction was further purified by HPLC on a reversed
phase silica gel (packing material C-18, 5 µm, pressure 2150 PSI,
temperature 40°C) eluted with water/acetonitrile (solvent gradient,
flow rate 1.5 ml/min). Manual collection led to the obtention of 8 major
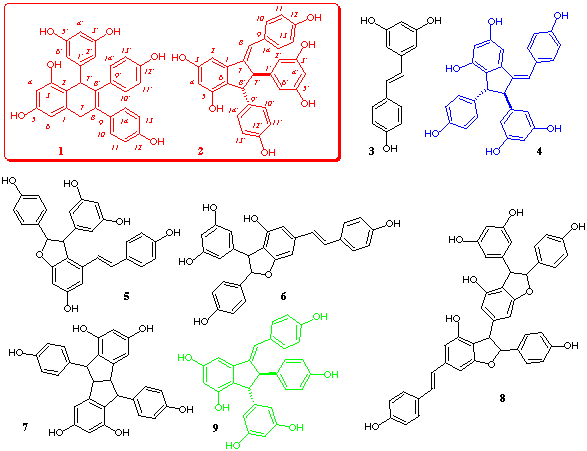
homogeneous fractions identified as cyphostemmin A 1 (35 mg/kg),
cyphostemmin
B 2 (22 mg/kg), resveratrol 3 (85 mg/kg), parthenocissin A [5]
4 (12 mg/kg), e-viniferin 5[6]
(90 mg/kg, trans -viniferin although, in some chromatography fractions,
a substantial amount of the corresponding cis isomer was also observed,
which has not been earlier described in the literature and has to be related
to another cis dehydrodimer of resveratrol recently obtained by incubation
of resveratrol in culture filtrates of B. cinerea [7]
; moreover, a slow transformation of this isomer into trans -viniferin
was observed on standing in solution), gnetin C 6 [8]
(90.3 mg/kg), pallidol 7 [9] (10.32
mg/kg) and a trimeric compound, gnetin E 8 [10]
(64.50 mg/kg). For economical reasons, we have worked on a small
quantity of crude-extract and the amount of material used for the identification
was thus about 1 mg for each compound ; therefore, we have not been able
to collect the 13C NMR data for all compounds.
The purified compounds were then subjected to classical spectroscopic
methodologies. First of all, the MS analysis allowed the rapid characterization
of resveratrol (M 228), of the various dimeric compounds (M 454) and of
the trimer (M 680).
open this scheme in a new window |
Results :
Structures of the two new compounds, cyphostemins A and B were inferred
from their spectroscopic data (NMR) through extensive NOE experiments and
through comparison with the spectroscopic data obtained for
parthenocissin A and Ampelopsin D 9
[11]. Antifungal activity and cytotoxicity
of this compounds have been evaluated as well as the biological activities
of an other resveratrol dimer obtained by enzimatic oligomerisation of
resveratrol (horse raddish peroxidase, H2O2).
References : follow this link
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with C0003 as the message subject of your e-mail.