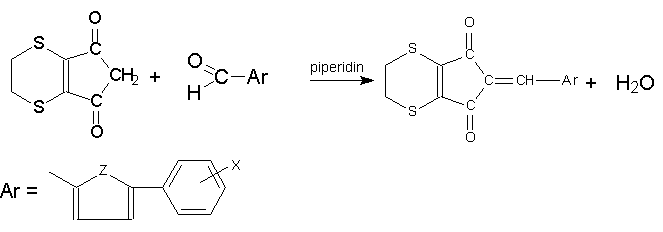
Synthesis
and antialgal effect of 2-substituted 5,6-dihydro-4,7-dithiaindane-1,3-diones
Ruzena Cizmarikova1, Katarina
Kralova2, Pavol Hrnciar3
1Department of
Chemical Theory of Drugs, Faculty of Pharmacy, Comenius University, Kalinciakova 8, SK-832 32 Bratislava, Slovakia., Tel.
421 7 50259315
E-mail: [email protected]
2Institute of
Chemistry and 3Department of Organic Chemistry, Faculty of Natural Sciences,
Comenius University, Mlynska dolina CH-2, SK-842 15 Bratislava, E-mail: [email protected]
Received: 6 August 2000 / Uploaded: 9 August
_______________________________________________________________________________________________
Abstracts
Derivatives
of indane-1,3-diones are compounds showing interesting properties not only from
theoretical view. Some 2-arylindane-1,3-diones are used as anticoagulants, dyes,
polymerization modifiers and rodenticides. This study deals with synthesis, spectral and
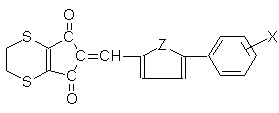
antialgal properties of new substituted 2-(5-aryl-2-thenylidene- or 2-
furfurylidene)-5,6-dihydro-4,7-dithiaindane-1,3-diones II.
Keywords: cyclic 1,3-diketone, indane-1,3-dione , dithiaindane-1,3-dione, condensation reaction, antialgal effect, Chlorella vulgaris
Introduction
Indane-1,3-diones
belong to the cyclic diketones. The presence of the adjacent carbonyl groups polarizes
methylenegroup , which can gives a lot of reaction of the C-acids
[1,2,3]. Among these
reaction belongs reaction with the carbonyl compounds [4,5] - the
Knoevenagel condensation.
2-(5-phenyl-2-furfurylidene
- or -2-thenylidene)-5,6-dihydro-4,7-dithiaindane-1,3-diones were prepared by synthesis
5,6-dihydro-4,7-dithiandane-1,3-dione with substituted 5-phenylthiophene-2-carbaldehyde or
5-phenylfurane-2-carbaldehyde, utilizing piperidine as catalyst. The other aim of this
study was to investigate the inhibition of chlorophyll production in the algal suspension
of Chlorela vulgaris .
Results and discussion
The obtained
compounds II (1t-9t., 1f-12f) with different colour intensity (from yellow to red) possessing high
melting points (Tables 1,2) were crystallized from
acetic acid or propan-2-one. The electronic spectra of II revealed three absorption bands with lmax = 210-215 nm,
260-280 nm, and 410-455 nm. For all the prepared compounds the position of the band at the
longest wavelengths (410-455 nm) was mostly influenced by the nature of substituent X. The
compounds bearing an electron-donating substituent on the phenyl ring in position 4 showed
a significant bathochromic shift. This was not observed for the compounds with the same
substituent in position 3. All compounds, independently on the position of substituent X
on the phenyl ring, were intensively coloured (Tables 1,2).
The compounds
II showed
two bands (Tables.1,2) in the region of the
stretching vibration of carbonyl group belonging to symmetrical and asymmetrical vibration
of the C=O group of the b-dicarbonyl
system.
In the 1H
NMR spectra the signal of the methylene group was at d = 3.34 ppm
(in the group SCH2CH2S) and 7.30-7.54 ppm (for =CH group) (Table 3).
For tested
compounds with Z = S the dependence of IC50
on the lipohilicity of substituent X was linear and the increase in the lipophilicity led
to the decrease in the antialgal activity against Chlorella
vulgaris. It can be assumed that this is connected with the restriction of the passage
of more lipophilic compounds through the hydrophilic regions of algal thylakoid membranes
and consecutive loss of their number reaching the site of inhibitory action. The compounds
with Z = O substituted in position 2 (X = 2-Cl, 2-NO2) exhibited rather lower
inhibitory activity than the comparable compounds with Z = S substituted in position 4.
The
previously prepared structurally similar benzylidene derivatives[4] did not affect
chlorophyll production in Chlorella vulgaris.
Thus, it can be concluded that for antialgal activity of the compounds the presence of
heteroatom (S, O) in the molecule is favourable (Table 4).
Experimental
The starting
material for both series was 5,6-dihydro-4,7-dithiaindane-1,3-dione I which
was prepared by the Gabriel modification of the Perkin synthesis from
5,6-dihydro-1,4,-dithiine-2,3-dicarboxylic anhydride [6] (Scheme 1). Treatment of I with substituted
5-phenylthiophene-2-carbaldehyde or 5-phenylfurane-2-carbaldehyde in 96 % ethanol,
utilizing piperidine as catalyst, afforded 2-(5-aryl-2-thenylidene)- or -2-furfurylidene)-5,6-dihydro-4,7-dithiaindane-1,3-diones
II. Under these conditions the aromatic
aldehydes reacted with I in the ratio 1: 1
(Scheme 2). The nitro derivatives, thus obtained, were reduced with SnCl2 in
acetic acid to the
corresponding amino derivatives. The Zeissel demethylation of the methoxy derivatives
using HBr acid gave the corresponding phenols[4].
Melting
points were determined by a Kofler hot bench and are uncorrected.
Electronic
spectra were measured on a spectrometer Hewlett-Paccard
8452A in methanol in the concentration 10-5 mol.dm-3. Infrared
spectra of prepared compounds were measured in the region 1800-1600 cm-1 on a
spectrophotometer FTIR IMPACT 400 D (Nicolet) as 10-2 mol.dm-3 con-centration in tetrachloromethane .
lH-NMR spectra
of prepared compounds dissolved in deuterochloroform were measured on spectrometer Tesla
BS-487 A with frequency 80 MHz using internal standard TMS.
Synthesis
of 2-substituted
4,7-dithia-5,6-dihydroindane-1,3-diones
II
Z= O, S X= 4-OCH3 , 4-CH3 , 3-CH3,
H, 2-Cl, 4-Cl, 4-Br, 3-F, 3-Br, 2-NO2, 3-NO2, 4-NO2
Into a flask
provided with a reflux condenser dithiaindandione (3.72 g, 0.02 mol), the appropriate
aldehyde (0.02 mole), and 98% ethanol (50-80ml) were added. After dissolving the
components, piperidine (2 drops) was added and reaction and reaction mixture with
substituted 5-phe-nylthiophene-2-carbaldehyde was refluxed for 10 min. Reaction with substituted 5 phenylfuryl-2-carbaldehyde
was carried at 200C for 10 minutes. Acetic acid (2 drops) was added into the
cooled solution and the precipitated crude product was crystallized from propan-2-one or
acetic acid.
Yield 70-90
%.
X= OH
The
metoxyderivatives (0.01 mol), acetic acid (15 ml), and 38% hydrobromic acid (40 ml) were
heated for 3 hrs under reflux. After cooling, hot water (250 ml) was added and crude
product was crystallized from acetic acid. Yield 65%.
X= NH2
Into a
three-necked flask fitted with a stirrer and condenser, tin chloride dihydrate (1.5 g),
acetic acid (10 ml), and hydrochloric acid (2 ml) were added. When tin dichloride was
dissolved, nitroderivatives (0.002 mol) was added and reaction mixture was stirred at 800
C for 2 hrs. After cooling, the salts of the corresponding amine precipitated and
was transferred into free amine by washing with ammonia. The crude product was
crystallized from propan-2-one. Yield 75%.
Antialgal effect
The effect of
these compounds from the prepared series on algal chlorophyll (Chl) production has been
investigated in statically cultivated Chlorella
vulgaris (photoperiod 16 h light/ 8h dark; illumination: 5 000 lx; pH = 7.2; Chl content at
beginning of cultivation: 0.5 mg dm-3) at room temperature according to [7].
Chl content of algal suspensions was extracted into N,N-dimethylformamide
and determined spectrophotometrically after 7 days of cultivation [8]. Because of low
water solubility of the tested compounds these were dissolved in dimethyl sulfoxide
(DMSO). The photosynthesis-inhibiting activity of II
was expressed by IC50 values, i.e.
by molar concentrations of inhibitors causing 50 % decrease of biological activity with
respect to the untreated control (Table 1).
Scheme
1
Synthesis of 5,6-dihydro-4,7-dithiaindane-1,3-dione I

Scheme
2 Synthesis of 2- substituted 5,6-dihydro-
4,7-dithiaindane-1,3-diones II -
The
Knoevenagel condensation
|
1t-9t.,
1f-14f
Z = O, S X= 4-OH, 4-OCH3, 4-CH3, 3-CH3,
3-NH2, H,
2-Cl, 4-Cl, 4-Br, 3-F, 3-Br, 2-NO2, 3-NO2, 4-NO2,
Table 1.
Characterization of prepared 2-(5-phenyl- 2-thenylidene) -
-5,6-
dihydro-4,7-dithiaindane-1,3-diones II
No. |
X |
Formula Mr |
M.p. oC Solvent |
lmax |
log e |
ns(C=O) |
nas(C=O) |
1t |
4-NH2 |
C18H13N
O3S3 371.50 |
236-7 a |
450 |
4.54 |
1723 |
1677 |
2t |
4-OCH3 |
C19H14O3S3 386.51 |
230-2 b |
445 |
4.54 |
1723 |
1679 |
3t |
4-CH3 |
C19H14O2S3 370,51 |
248-9 b |
436 |
4.36 |
1725 |
1678 |
4t |
3-CH3 |
C19H14O2S3 370.51 |
180-2 b |
430 |
4.39 |
1725 |
1679 |
5t |
-H |
C18H12O2S3 356,49 |
201-3 b |
430 |
4.43 |
1725 |
1679 |
6t |
4-Cl |
C18H11O2S3
Cl 390,93 |
244-6 b |
425 |
4.06 |
1725 |
1680 |
7t |
4-Br |
C18H11O2S3
Br 435,39 |
254-6 b |
442 |
4.03 |
1726 |
1680 |
8t |
3-Br |
C18H11O2S3
Br 435,39 |
203-5 b |
432 |
4.07 |
1726 |
1680 |
9t |
4-NO2 |
C18H11O4NS3 401,48 |
280-2 b |
413 |
4.08 |
1726 |
1682 |
a-propan-2-one
., b- acetic acid e in l.mol-1.cm-1 n in cm-1
lmax
in nm
Table
2. Characterization of the prepared 2-(5-phenyl-2-furfurylidene)-
-
5,6- dihydro-4,7-dithiaindane-1,3-dione II
No. |
X |
Formula Mr |
M.p.0C Solvent |
lmax |
log e |
ns(C=O) |
nas(C=O) |
1f |
4-OH |
C18H12O4S2 340.42 |
268-9 a |
455 |
4.57 |
1721 |
1675 |
2f |
4-OCH3 |
C19H14O4S2 356.42 |
203-5 b |
450 |
4.65 |
1722 |
1676 |
3f |
4-CH3 |
C19H14O4S2 354.45 |
263-5 b |
433 |
4.66 |
1724 |
1677 |
4f |
3-NH2 |
C18
H13 O3NS2 355.44 |
218-9 b |
432 |
4.63 |
1724 |
1676 |
5f |
-H |
C18H12O3S2 340.42 |
243-5 b |
430 |
4.62 |
1724 |
1677 |
6f |
4-Cl |
C18H11O3S2Cl 374.87 |
274-5 b |
432 |
4.06 |
1724 |
1677 |
7f |
4-Br |
C18H11O3S2Br 479.32 |
267-9 b |
435 |
4.11 |
1724 |
1678 |
8f |
3-F |
C18H11O3S2F 418.42 |
245-7 b |
432 |
4.47 |
1715c |
1678c |
9f |
3-Cl |
C18H11O3S2Cl 374.87 |
244-6 b |
427 |
4.48 |
1725 |
1678 |
10f |
3-Br |
C18H11O3S2Br 479.32 |
254-5 b |
430 |
4.38 |
1725 |
1679 |
11f |
3-NO2 |
C18H11NO5S2 385.42 |
310-2 a |
425 |
4.36 |
1718c |
1673c |
12f |
4-NO2 |
C18H11NO5S2 385.42 |
314-6 a |
442 |
4.58 |
1719c |
1674c |
13f |
2-NO2 |
C18H11NO5S2 385.42 |
228-230 a |
426 |
5.46 |
1719 |
1675c |
14f |
2-Cl |
C18H11O3S2Cl 374.87 |
214-5 a |
428 |
4.29 |
1720 |
1670 |
a -
propan-2-one., b- acetic acid c-for the low
solubility spectrum was measured in chloroform
The results
of elementary analyses (C,H,S,X) are in agreement with the calculated values.
Table
3. 1H-NMR spectra
Z |
X |
d
(ppm) |
|||
S(CH2)2S |
=CH |
CHarom. |
X
(H) |
||
S |
-H |
3.34 4H |
7.35 1H |
7.40-7.88 7H |
|
S |
4-Br |
3.34 4H |
7.54 1H |
7.38-7.85 6H |
|
S |
4-OCH3 |
3.34 4H |
7.30 1H |
7.54-7.85 6H |
3.38 3H |
S |
4-CH3 |
3.34 4H |
7.32 1H |
7.54-7.86 6H |
2.38 3H |
S |
4-NO2 |
3.34 4H |
7.49 1H |
7.60-8.40 6H |
|
O |
2-Cl |
3.37 4H |
7.37 1H |
7.33-8.23 6H |
|
O |
2-NO2 |
3.31 4H |
7.31 1H |
7.33-8.26 6H |
|
Table
4. Inhibition of chlorophyll in Chlorella vulgaris
Z |
X |
IC50 [ mmol . dm-3] |
Z |
X |
IC50 [ mmol . dm-3] |
S |
-H |
46.6 |
O |
2-Cl |
97.9 |
S |
4-Cl |
71.4 |
O |
2-NO2 |
88.6 |
S |
4-Br |
74.9 |
O |
4-Br |
84.2 |
S |
4-CH3 |
53.3 |
O |
4-OH |
131.4 |
S |
4-OCH3 |
42.1 |
O |
4-CH3 |
53.5 |
S |
4-NO2 |
52.3 |
|
|
|
Acknowledgements. Our thank are
due to prof. Fisera and prof. Krutosikova for the supply of substituted
5-phenyl-2-thiophenecarbaldehyde and 5-.phenyl-2-furancarbaldehyde used in the preparation
of the compounds under study.
This study was supported by Scientific Grant Agency of Ministry of Education, Slovak Republic ( grants No. 1/ 7277/20 and 1/7262/20)
References
1. Artico, M.; Di Santo, R.; Costi,
R..; et al.; J.Med. Chem. 1998,41, 3948.
2. Sarhan, A,A.; Afsah, E,M.; Abdelaal, M,Y.; Ibrahim, M,R.; React. Funct. Polym. 1997, 32, 231.
3. Cizmarikova
,R.; Hrnciar, P.; Cupkova, V.; 1990 CS 276 674
4. Hrnciar P.;
Cizmarikova R.; Chem. Zvesti 1975, 29,
836-842.
5. Perjessy,
A., Hrnciar, P., Frimm, R., Fisera, L.: Tetrahedron
1972, 28, 3781
6. Hrnciar P.;
Sokolova R.; Monatsh Chem. 1973, 104, 1224-1230.
7. Kralova K.; Sersen F.; Melnik M.; J. Trace Microprobe Techn. 1998, 16, 491.
8. .Inskeep W.
P., Bloom P. R., Plant Physiol. 1985, 77, 483.