
Facile and Efficient Synthesis of
Functionalized Asymmetric Porphyrins
with Organolithium Reagents
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[C0010]
|
Facile and Efficient Synthesis of |
 |
Xiangdong Feng and Mathias O. Senge*
Institut für Chemie, Organische Chemie, Freie Universität Berlin, Takustr. 3, D-14195 Berlin, Germany
Fax: 49-(0)-30-838-55163, E-mail: [email protected]
Received: 6 August 2000 / Uploaded: 9 August
Abstract:
Functionalized asymmetric porphyrins bearing highly reactive centers in substituents at the meso-positions were synthesized in good yields by the reaction of 5,15-diphenylporphyrin with organolithium reagents. Using these porphyrins as precursors highly complex tetrapyrrolic systems are accessible. The synthetic strategy is based on our earlier reports on the combined use of organolithium and alkyliodide reagents for the functionalization of porphyrins [1].Introduction
Tetrapyrroles have broad technical applications in the area of catalysis [2], nonlinear optics [3], receptors for molecular recognition [4], photosensitizers [5], and medicinal applications (PDT). Further development of these applications requires asymmetric and highly functionalized compounds. For these, novel and efficient synthetic methods, preferentially via a simple modification of easily accessible symmetric porphyrins have to be developed. Thus, functionalized asymmetric porphyrin precursors like 1 are key intermediates for the required compounds.
 Unfortunately the porphyrin skeleton is very resilient towards many reactions that should proceed quite easily on paper like Friedel-Crafts or Grignard reactions and so far, only a few efficient functionalization reactions have been described for porphyrins [6,7]., Especially, the direct, regioselective introduction of functional groups was rather limited up to now.
Unfortunately the porphyrin skeleton is very resilient towards many reactions that should proceed quite easily on paper like Friedel-Crafts or Grignard reactions and so far, only a few efficient functionalization reactions have been described for porphyrins [6,7]., Especially, the direct, regioselective introduction of functional groups was rather limited up to now.
In general, there are three synthetic ways to functionalized, asymmetric porphyrins:
Due to the difficult work up and unsatisfactory yields of a) and the limited applicability of b) only c) offers some potential for further developments. Known functionalization reactions included Vilsmeier formylation, nitration and halogenation reactions [9].. However, will the later offer potential in metal assisted C-C coupling reactions many of these modification reactions require harsh conditions and/or use of metalloporphyrins. Subsequent demetallation again, requires harsh conditions all resulting in unsatisfactory yields and low tolerance towards functional groups.
We have started a program aimed at the development of highly efficient methods for the functionalization and modification of porphyrins. Two years ago, we found that porphyrins readily undergo meso substitution reactions with organolithium reagents [10] and since then have developed this reaction to a generally applicable method for the for the preparative synthesis of the functionalized asymmetric porphyrin precursors. Here, we will report several facile and efficient applications of this methodology to the synthesis of highly functionalized porphyrins methods and demonstrate their active chemical properties in coupling reactions.
Results and Discussion
Porphyrins react readily at the meso positions with organolithium reagents. The overall reaction is a nucleophilic substitution and proceeds via initial reaction of the organic nucleophile with a meso carbon yielding an anionic species, which is hydrolyzed to a dihydroporphyrin (porphodimethen or phlorin). Subsequent oxidation with DDQ yields meso substituted porphyrins. The reaction is highly versatile as it is accomplished in high, often quantitative, yields with various alkyl or aryl lithium reagents and can be performed both with metalloporphyrins and its free base porphyrins. In addition, the anionic intermediates obtained from reaction of LiR with nickel(II)porphyrins can be used as in situ nucleophiles for the reaction with alkyl iodides. Thus, introduction of two different substituents (at the 5- and 15-position) in a one-pot reaction is possible [1].
5,15-Diphenylporphyin (Scheme 1) is an easily synthesized symmetric porphyrin [11] with two free meso positions, in which new functionalized substituents from organolithium reagents and alkyliodides can be selectively induced without attack at ß positions [12]. In addition, repetition of the addition-oxidation sequence allows the synthesis of bifunctionalized asymmetric porphyrins by introducing a second functional group without formation of regioisomers as is observed in similar reactions with octaethylporphyrin [13]. Thus,
we used 5,15-diphenylporphyrin for the preparation of various monofunctionalized porphyrins (Scheme 1).
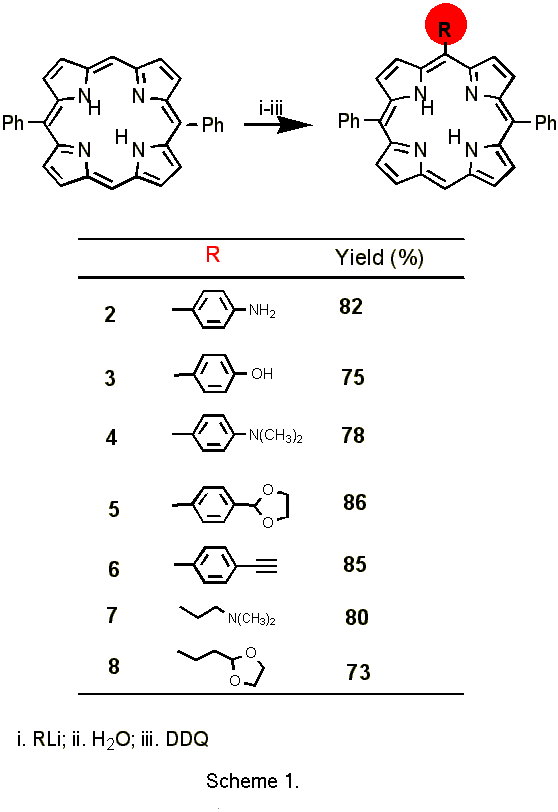
Furthermore, these porphyrin precursors can be readily refunctionalized to more useful reactive porphyrin pigments. In this context, we report here several new porphyrins, which were obtained from the precursor compounds described above through the reaction of the porphyrin anionic intermediate with alkyl iodides.1 The results are shown in Scheme 2.

In order to test the chemical reactivity of the functionalized porphyrins synthesized, we subjected them to coupling reactions to obtain novel porphyrins suitable for the applications described in the introduction. E. g. porphyrins 11-14, 16-17, 19 posses the amphiphilic properties often used in membrane studies [19], porphyrin 15 is a redox-system and exhibits interesting electrochemical properties [20], porphyrin 10 can be easily bound to gold particles and layer for surface studies [21], etc.
Thus, the present methodology in conjunction with established techniques should allow the synthesis of almost any desired meso-functionalized porphyrin and more complicated macrocyclic systems with special chemical and physical properties in few steps and with high yields.
Acknowledgments:
This work was supported by grants from the Deutsche Forschungsgemeinschaft (Se543/2-5 and Heisenberg fellowship /3-2) and the Fonds der Chemischen Industrie.References
[1] Feng, X.; Senge, M. O., Tetrahedron 2000, 56, 587.
[2] Montanari, F.; Casella, L. (Eds.), Metalloporphyrins Catalyzed Reactions, Kluwer Academic Press, Dordrecht, 1994.
[3] Su, W.; Cooper, T. M.; Brant, M. C., Chem. Mat. 1997,10, 1212.
[4] Aoyama, Y.; Yamagishi, A; Asagawa, M.; Toi, H.; Ogoshi, H., J. Am. Chem. Soc. 1988, 110, 4076.
[5] Ali, H.; Lier, J. E., Chem. Rev. 1999, 99, 2379.
[6] Vicente, M. G. H.; Rezzano, I. N.; Smith, K. M. Tetrahedron Lett. 1990, 31, 1365-1368.
[7] Vicente, M. G. H., in The Porphyrin Handbook, Vol. 1., Kadish, K. M.; Smith K. M.; Guilard R (Eds.), Academic Press: San Diego, 2000; pp. 150.
[8] Lindsey, J. S., in The Porphyrin Handbook, Vol. 1. Kadish, K. M.; Smith K. M.; Guilard R (Eds.), Academic Press: San Diego, 2000; pp. 45.
[9] Fuhrhop, J., in Porphyrins and Metalloporphyrins, Smith, K. M. (ed.), Elsevier Scientific Publishing Company: Amsterdam, 1975, pp. 625.
[10] Kalisch, W. W., Senge, M. O. Angew. Chem. Int. Ed. Engl. 1998, 37, 1107. Senge, M. O.; Kalisch, W. W.; Bischoff, I., Chem. Eur. J. 2000, in press.
[11] Brückner, C.; Posakony, J. J.; Johnson, C. K.; Boyle, R. W.; James, B. R.; Dolphin, D., J. Porphyrins Phthalocyanines 1998, 2, 455.
[12] Senge, M. O.; Feng, X., Tetrahedron Lett. 1999, 40, 4165.
[13] Senge, M. O.; Bischoff, I., in preparation.
[14] Wennerstrom, O.; Ericsson, H.; Raston, I.; Svensson, S.; Pimlott, W., Tetrahedron Lett. 1989, 30, 1129.
[15] Sessler, J. L.; Capuano, V. L.; Harriman, A. J., J. Am. Chem. Soc. 1993, 115, 4618.
[16] Pandey, R. K.; Smith, N. W.; Shiau, F-Y.; Dougherty, T. J.; Smith, K. M., J. Chem. Soc., Chem, Commun. 1991, 1637.
[17] Osuka, A.; Ikeda, M.; Shiratori, H.; Nishimura, Y.; Yamazaki, I., J. Chem. Soc., Perkin Trans. 2, 1999, 1019.
[18] Wagner, R. W.; Johson, T. E.; Lindsey, J. S., J. Am. Chem. Soc. 1996, 118, 11166.
[19] Amao, Y; Asai, K.; Miyakawa, K.; Okura, I., J. Porphyrins Phthalocyanines 2000, 4, 19.
[20] Manka, J.S.; Lawrence, D. S., Tetrahedron Lett. 1989, 30, 6989.
[21] Gryko, D. T.; Clausen, C.; Lindsey, J. S., J. Am. Chem. Soc. 1999, 121, 8635.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with C0010 as the message subject of your e-mail.