[C0014]
Synthesis and Structural Properties of Neoglycolipids Anchored at Membrane Surfaces
Christian Gege, Momo Arsic, Richard R. Schmidt and Armin Geyer
Fachbereich Chemie, Universität Konstanz, 78457 Konstanz, Germany
Received: 7 August 2000 / Uploaded: 10 August
Aims and Scope
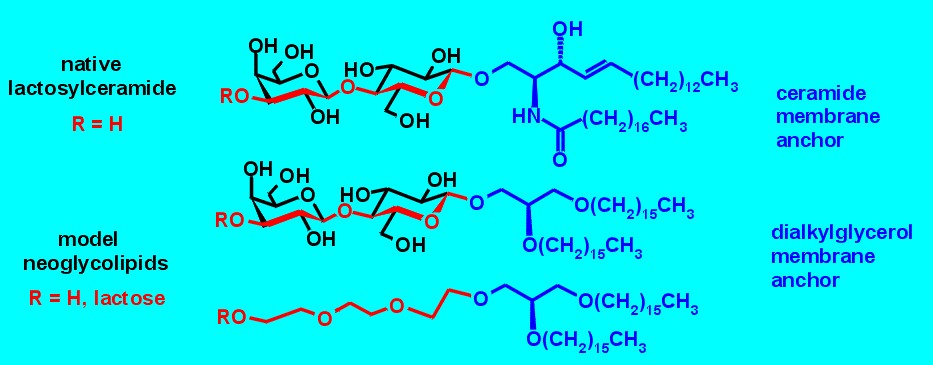
Glycolipids cover the surface of living cells and determine their properties in cell-adhesion processes. The extremely complex and heterogeneous native cell membrane forbids the functional characterization of individual glycolipid components. Homogeneous neoglycolipids on model membranes reduce the complex native glycocalix to its essential features.

Figure 1. 1,2-di-O-alkyl-sn-glycerol serves as the membrane anchor for lactose building blocks. The triethyleneglycol spacer mimics one lactose disaccharide. Although the number of bonds are the same (red), the flexibility of the triethyleneglycol spacer is much higher.
Synthesis of the 1,2-di-O-alkyl-sn-glycerol membrane anchor
Fig. 2 gives an outline of the synthetic route to the 1,2-di-O-hexadecyl-sn-glycerol derivatives Pegn (n = 3, 6, and 9).3 Benzyl protected triethylene glycol derivative 1 was synthesized in one step from commercially available triethylene glycol monochlorohydrin. The spacered lipid Peg3 was synthesized in high overall yield by the coupling of 1 (2.5 equivalents) and 1,2-O-isopropylidene-sn-glycerol in the presence of sodium hydride as base and tetrabutylammonium iodide (TBAI) as activator, followed by acid catalyzed de-O-isopropylidenation, di-O-alkylation with hexadecyl bromide under the above described conditions, and finally hydrogenolytic O-debenzylation. Repetitive coupling of Peg3 with the building block 1 (2.5 equivalents) under the above described conditions yielded, after de-O-benzylation, the elongated lipids Peg6 and Peg9.
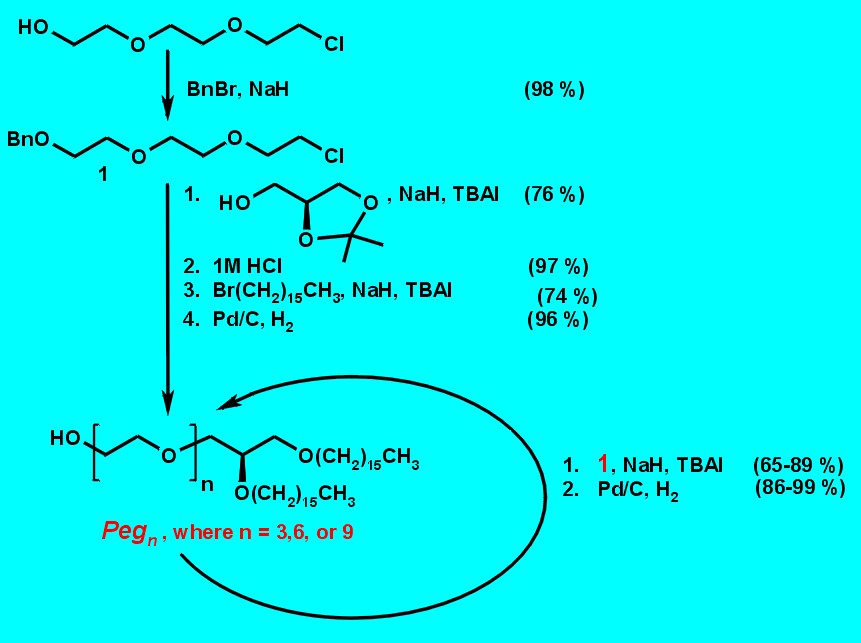
Figure 2. Synthesis of ethylene glycol spacered 1,2-Di-O-hexadecyl-sn-glycerol derivatives Pegn (n = 3,6, and 9).
Attachment of lactose or oligolactose
The synthetic route to these glycolipids is shown in Fig. 3. Glycosylation of known lactose trichloroacetimidate 21 with 1,2-di-O-hexadecyl-sn-glycerol2,3 as lipid anchor was performed in the presence of BF3�Et2O as catalyst to afford the lactose intermediate 3 in 94 % yield. The b-configuration of the newly formed glycosidic bond was confirmed in the 1H NMR spectrum (J1,2 = 7.9 Hz). Acid-catalyzed cleavage of the isopropylidene group furnished 4, which served as acceptor for the next glycosylation. Removal of the O-benzoyl protective groups with sodium methoxide in CH2Cl2/CH3OH afforded Lac1 in almost quantitative yield after purification on reversed phase (RP-18) silica gel.
Elongation of 4 with donor 2 in the presence of BF3�Et2O as catalyst at � 20�C afforded exclusively the expected b(1®3)-connected bis-lactose intermediate 5 (J1,2 = 7.9 Hz for 1c-H; shift of the 4b-H-signal from 3.85 ppm to 5.29 ppm after acetylation). Again, de-O-isopropylidenation furnished the following acceptor 6, and de-O-benzoylation the desired Lac2 derivative under the conditions described above. Repetitive coupling of 6 with donor 2 yielded the elongated protected glycolipid 7, which was de-O-isopropylidenated in the same manner. Due to the low solubility of Lac3 the de-O-benzoylation and purification was performed in dimethyl sulfoxide (DMSO) mixtures.
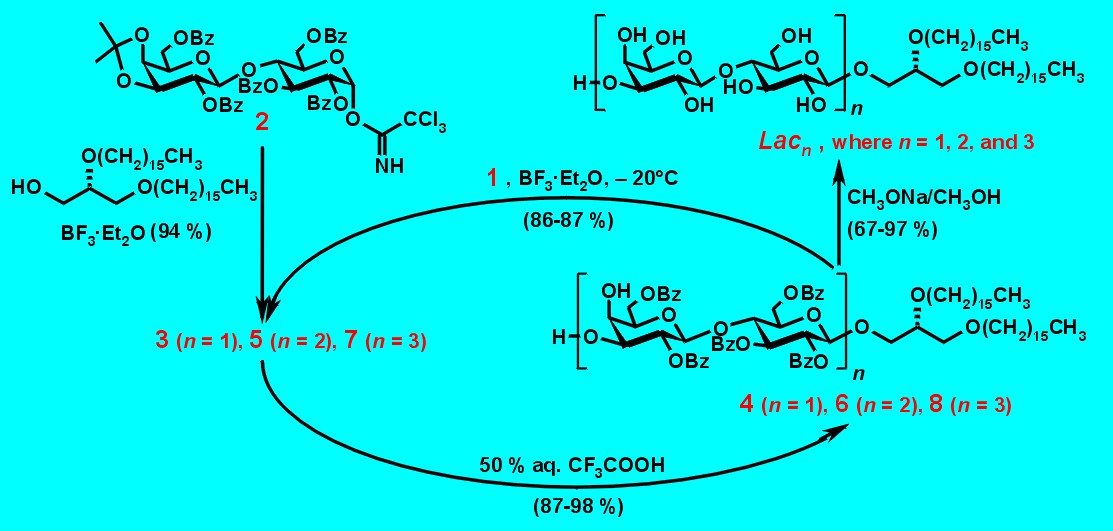
Figure 3. Synthesis of Lacn (n = 1, 2, and 3) by repetitive glycosylation.
Fig. 4 shows mixed neoglycolipids characterized by triethylene spacers of various lengths inserted between lactose and the membran anchor. They were named LacPegn, with n corresponds to the number of ethylene glycol units (n = 3, 6, or 9). The benzoylated lactose trichloroacetimidate 9 is available from lactose in a three-step procedure.4 Again, the benzoyl protecting group next to the anomeric activation directs the formation of b-linked glycolipids without formation of undesired orthoester.
Glycosidation of 1,2-di-O-hexadecyl-sn-glycerol derivatives of Pegn (n = 3, 6, 9) as acceptors was performed with donor lactose trichloroacetamidate 9 in the presence of TMSOTf as catalyst to afford the protected products 10, 11, and 12, respectively. The newly formed glycosidic bond was found exclusively b -oriented in the 1H-NMR spectrum. Removal of the benzoyl groups with sodium methoxide in CHCl3/CH3OH affored the LacPegn derivatives in quantitative yields after purification by flash chromatography.
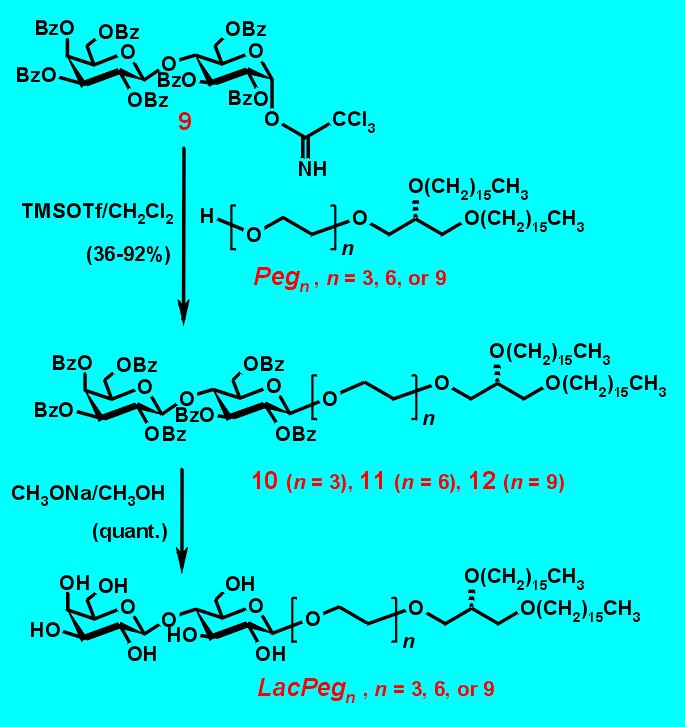
Figure 4. Synthesis of the spacered lactosyl-glycolipids.
Synthesis of a neoglycolipid with a deuterated acetyl group
Glycosylation of Peg9 with the glucosamine imidate5 led to b-linked glycoside (J1,2 = 8.6 Hz) in high yield. Treatment with activated zinc in deuterated acetic anhydride led to the N-acetyl derivative, which furnished on treatment with NaOMe in MeOH the desired glucosamine derivative GlcNAcPeg9.3
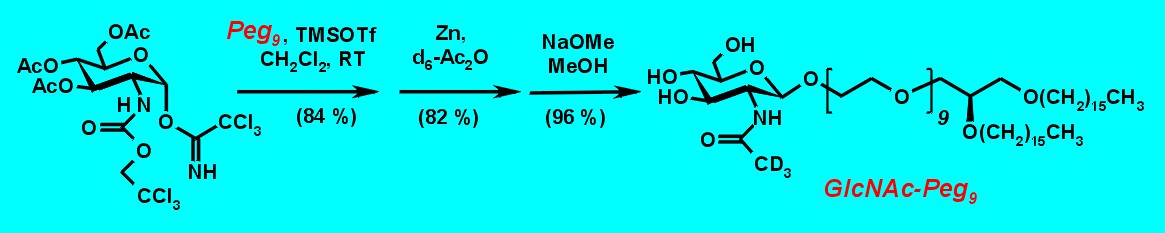
Figure 5: Synthesis of GlcNAcPeg9.
NMR studies
So-called bicelles are a powerfull tool for the spectroscopic characterization of neoglycolipids. This membrane model was developed by Prestegard for NMR studies.6 Bicelles combine several advantageous qualities. They form a flat and highly solvated bilayer model which is stable between 303 and 320 K and which can be studied with highfield NMR methods (Fig. 6). Fig. 7 shows typical phase transitions when glycolipid GlcNAcPeg9 is inserted into the membranes. In the temperature range of bicelle formation, two 31P NMR signals are observed which belong to the edge (lowfield, mainly dihexanoylphosphatidylcholine, DHPC) and the flat bilayer (highfield, mainly dimyristoylphosphatidylcholine, DMPC). The 1H NMR shows excessive line-broadening. Also the carbohydrate protons resonances are broadened, proving the slowing down of the glycolipid tumbling. The temperture of phase transition is influenced by the admixed glycolipid. 31P NMR spectra and 1H NMR spectra are shown in Fig. 7 for GlcNAcPeg9. Only in a very small region of less than 5 K the bicelles are formed. The 31P NMR spectra exhibit two resonances: the broader highfield signal originates from the flat bilayer, while the sharper lowfiled signal comes from the DHPC at the edges of the bicelles. Only one isotropic 31P resonance is detected above and below this temperature range. At 302 K all three signals are visible and indicate the existence of bicelles plus isotropic liposomes. A very sharp phase transition is observed between 305 K and 306 K, the bicelles `melt�.

Figure 6: Disc-shaped micelles, so-called bicelles are formed by DMPC and DHPC. Flat lipid bilayers are formed by the DMPC fraction, while the DHPC molecules probably concentrate at the edges of the bicelles. The bicelles form a nematic fluid-crystalline phase with the average disc vector perpendicular to the direction of the magnetic field B0. The diameter of the disc varies from 10 to 100 nm, the thickness is about 4 nm.

Figure 7: Temperature dependence of a 3:1 mixture of DMPC and DHPC containing the neoglycolipid GlcNAcPeg9 in a mM concentration.
Conclusion
The amphiphilic lactose-derived neoglycolipids described here exhibit poor solubilities in monolayered or bilayered membrane models. The triethylene spacer improves the solubilities. Although we described a straightforward synthetic strategy making the final compounds accessible in the gram scale, their NMR spectroscopic characterization in flat phospholipid bilayers remains a difficult task.
References
(1) L. Lay, R. Windmüller, S. Reinhardt, R. R. Schmidt, Carbohydr. Res. 1997, 303, 39.
(2) O. H. Abdelmageed, R. I. Duclos jr., R. G. Griffin, D. J. Siminovitch, M. J. Ruocco, A. Makriyannis, Chem. Phys. Lipids 1989, 50, 163.
(3) C. Gege, G. Bendas, J. Vogel, U. Rothe, R. R. Schmidt, Chem. Eur. J. 2000, 6, 111.
(4) G. J. P. H. Boons, F. L. van Delft, P. A. M. van der Klein, G. A. van der Marel, J. H. van der Boom, Tetrahedron 1992, 48, 885.
(5) W. Dullenkopf, J. C. Castro-Palomino, L. Manzoni, R. R. Schmidt, Carbohydr. Res. 1996, 296, 135.
(6) C. R. Sanders II, B. J. Hare, K. P. Howard, J. H. Prestegard, Prog. NMR Spectrosc. 1994, 26, 421; J. H. Prestegard, Nature, Struct. Biol. NMR II Supplement 1998, 5, 517; P. J. Bolon, J. H. Prestegard, J. Am. Chem. Soc. 1998, 120, 9366.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with C0014 as the message subject of your e-mail.