Synthesis and Biological Activity of
L. Kubicova1, H. Dostal1, J. Kunes2, K. Kralova3, V. Buchta4, J. Kaustova5, K. Waisser1
Department of Inorganic and Organic Chemistry1, Laboratory of Structure and Interactions of Biologically Active Molecules2, Department of Biological and Medical Sciences4, Faculty of Pharmacy in Hradec Kralove, Charles University in Prague, Heyrovskeho 1203, 500 05 Hradec Kralove, Czech Republic. Tel. +420 49 5067339, Fax +420 49 5210002. E-mail: [email protected]
Institute of Chemistry3, Faculty of Natural Sciences, Comenius University, Mlynska dolina CH-2, 842 15 Bratislava, Slovak Republic. E-mail: [email protected]
National Reference Laboratory for Mycobacterium Kansasii5, Institute of Hygiene, 728 92 Ostrava, Czech Republic
Received: 7 August 2000 / Uploaded: 10 August
Abstract: A series of substituted 2-amino-N-phenylbenzamides (1) and 3-phenyl-1,2,3-benzotriazin-4(3H)-ones (2) was synthesized and evaluated in vitro for their antimycobacterial, antifungal, and photosynthesis-inhibiting properties. Some compounds 1 exhibited good activity against Mycobacterium tuberculosis as well as against atypical strains of mycobacteria. The introduction of chloro substituent into position 5 improved the antimycobacterial activity of 1. Most 5-chloro derivatives 1 were more active against atypical strains than INH. The most active derivative was 2-amino-5-chloro-N-(4-sec-butylphenyl)benzamide. A moderate antifungal activity against Trichophyton mentagrophytes was found for some 1, but significant activity against the other tested fungal strains was not observed.
In general, the ring closure in 3-phenyl-1,2,3-benzotriazin-4(3H)-ones (2) led to decrease or loss of antimycobacterial as well as antifungal activity. The photosynthesis-inhibiting activity of 2 concerning inhibition of oxygen evolution rate in spinach chloroplasts was investigated. The relatively low photosynthesis-inhibiting activity of 2 is probably a consequence of their low aqueous solubility causing a restricted passage of the inhibitor through the hydrophilic regions of thylakoid membranes.
Keywords: 2-amino-N-phenylbenzamides, 3-phenyl-1,2,3-benzotriazin-4(3H)-ones, antimycobacterial activity, antifungal activity, photosynthesis-inhibiting activity, mycobacteria, fungi, spinach chloroplasts.
Introduction
The development of new antimycobacterial agents is presently of utmost importance and should proceed at a rapid pace, because of the return of tuberculosis and other mycobacterial diseases during the past decade. 3-Phenyl-2H-1,3-benzoxazine-2,4(3H)-diones represent a novel group of antimycobacterially active compounds which exhibit a strong in vitro antimycobacterial activity against Mycobacterium tuberculosis, M. kansasii, and M. avium [1--4], as well as a significant antimycotic activity [2]. The substitution by halogen in position 6 of heterocyclic moiety of the molecule lead to increase of antimycobacterial activity [2]. In some groups of heterocyclic compounds, the aza derivatives (e. g. 2,3-dianilinoquinoxalines) were much more effective against mycobacteria than their isosteric oxa and thia analogues [5]. This fact prompted us to investigate isosteric aza analogues of 3-phenyl-2H-1,3-benzoxazine-2,4(3H)-diones, i. e. 3-aryl-1,2,3-benzotriazin-4(3H)-ones. In the group of 3-substituted-1,2,3-benzotriazin-4(3H)-ones, some biologically active derivatives are known which possess hypnotic [6], anticonvulsive [6,7], analgesic [8], antisecretory [9], or antifungal [10] activity. Derivatives of various heterocyclic compounds were found to be inhibitors of the photosynthetic electron transport in photosynthesizing organisms [11--16]. It was found that the presence of carbamoyl group in the molecule of the inhibitor is favourable for photosynthesis-inhibiting activity [11, 16]. 3-Phenyl-2H-1,3-benzoxazine-2,4(3H)-diones and some their aza analogues, e.g. 3-phenylquinazolin-4(3H)-ones substituted on the 3-phenyl ring, inhibit the chlorophyll production in statically cultivated alga Chlorella vulgaris [17].
Biological activity of 2-amino-N-phenylbenzamides synthesized as intermediates for 3-phenyl-1,2,3-benzotriazin-4(3H)-ones is also interesting. In our previous study, we found that 2-amino-N-(3,4-dichlorophenyl)benzamide was the most antimycobacterially active derivative from 2-amino-N-phenylbenzamides unsubstituted in the acyl moiety [3]. Isomeric 2-phenylaminobenzoic acids possess also in vitro antimycobacterial activity, the activity is enhanced in the 5-chloro derivatives [18]. 2-Amino-N-pnenylbenzamide showed antifungal activity against phytopathogenic fungi [19]. From these results we concluded that antimycobacterial activity of 2-amino-N-phenylbenzamides could be enhanced by the introduction of chloro substituent into position 5 and that the coumpounds could be active against fungi. 2-Amino-N-phenylbenzamides also inhibit photosynthetic electron transport in spinach chloroplasts and chlorophyll production in Chlorella vulgaris [20].
We now describe the synthesis of 2-amino-N-phenylbenzamides (1) and 3-phenyl-1,2,3-benzotriazin-4(3H)-ones (2) and report on the in vitro evaluation of their antimycobaterial, antifungal and photosynthesis-inhibiting activity. The influence of structural modifications to these activities is also discussed.
Results and Discussion
Chemistry
2-Amino-N-phenylbenzamides (1a--1k) were prepared from 2H-3,1-benzoxazine-2,4(1H)-dione by the reaction with the corresponding anilines in glacial acetic acid under reflux. The reaction mixtures were poured into water, the products were filtered off and crystallized from aqueous ethanol. 2-Amino-5-chloro-N-phenylbenzamides (1l--1t) were synthesized by melting of 6-chloro-2H-3,1-benzoxazine-2,4(1H)-dione with the appropriate aniline at 170--180 oC and isolated by column chromatography on silica gel using mixtures of petroleum ether with ethyl acetate. 3-Phenyl-1,2,3-benzotriazin-4(3H)-ones (2) were obtained from compounds 1 by diazotization followed by spontaneous cyclization and crystallized from aqueous ethanol. The syntheses are outlined in Scheme 1. The characteristic data of compounds 1 and 2 are given in Table 1 and Table 2.
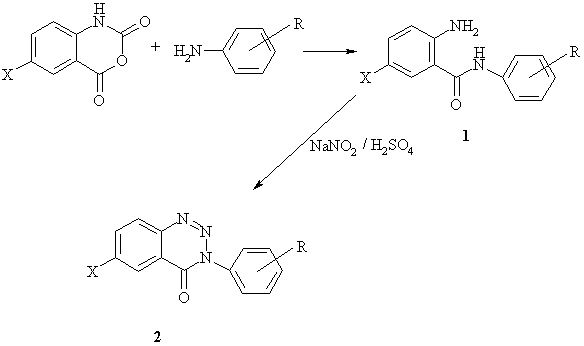 X = H; Cl R = H; 4-Cl; 4-Br; 4-F; 4-CH3; 4-isoC3H7; 4-C4H9; 4-sec-C4H9; 4-OCH3; 3-Cl; 3-F; 3,4-Cl2; 3,4-(CH3)2 |
Table 1. Physical properties and analytical data of the compounds
| Compd. | Formula (M. w.) |
X | R | M. p. (oC) (Yield, %) |
% Calc. % Found |
||
| C | H | N | |||||
| 1a | C13H12N2O (212.2) |
H | H | 130--131a) (80) |
-- | -- | -- |
| 1b | C13H11ClN2O (246.7) |
H | 4-Cl | 144--145a) (76) |
-- | -- | -- |
| 1c | C13H11BrN2O (267.1) |
H | 4-Br | 155--156a) (82) |
-- | -- | -- |
| 1d | C13H11FN2O (216.2) |
H | 4-F | 131--132a) (75) |
-- | -- | -- |
| 1e | C14H14N2O (226.3) |
H | 4-CH3 | 150--151a) (84) |
-- | -- | -- |
| 1f | C17H20N2O (268.4) |
H | 4-C4H9 | 205--207 (81) |
76.09 75.99 |
7.51 7.48 |
10.44 10.41 |
| 1g | C14H14N2O2 (242.3) |
H | 4-OCH3 | 126--127a) (77) |
-- | -- | -- |
| 1h | C13H11ClN2O (246.7) |
H | 3-Cl | 136--137a) (79) |
-- | -- | -- |
| 1ib) | C13H11FN2O (216.2) |
H | 3-F | 118--120 (76) |
67.82 67.69 |
4.82 4.65 |
12.17 12.24 |
| 1j | C13H10Cl2N2O (281.1) |
H | 3,4-Cl2 | 145--146a) (77) |
-- | -- | -- |
| 1k | C15H16N2O (240.3) |
H | 3,4-(CH3)2 | 115--116a) (77) |
-- | -- | -- |
| 1l | C13H11ClN2O (246.7) |
Cl | H | 153--154a) (39) |
-- | -- | -- |
| 1m | C13H10Cl2N2O (281.1) |
Cl | 4-Cl | 174--175a) (29) |
-- | -- | -- |
| 1n | C13H10BrClN2O (325.6) |
Cl | 4-Br | 189--191a) (15) |
-- | -- | -- |
| 1o | C14H13ClN2O (260.7) |
Cl | 4-CH3 | 179--181a) (63) |
-- | -- | -- |
| 1p | C16H17ClN2O (288.8) |
Cl | 4-isoC3H7 | 136--138 (53) |
66.55 66.76 |
5.93 6.08 |
9.70 9.55 |
| 1r | C17H19ClN2O (302.8) |
Cl | 4-sec-C4H9 | 115--118 (45) |
67.43 67.83 |
6.32 6.27 |
9.25 9.17 |
| 1s | C13H9Cl3N2O (315.6) |
Cl | 3,4-Cl2 | 163--165a) (27) |
-- | -- | -- |
| 1t | C13H10ClFN2O (264.7) |
Cl | 4-F | 168--170 (20) |
58.99 58.70 |
3.81 3.79 |
10.58 10.22 |
| 2a | C13H9N3O (223.2) |
H | H | 152--154a) (77) |
-- | -- | -- |
| 2b | C13H8ClN3O (257.7) |
H | 4-Cl | 185--186a) (76) |
-- | -- | -- |
| 2c | C13H8BrN3O (302.1) |
H | 4-Br | 204--206a) (72) |
-- | -- | -- |
| 2d | C13H8FN3O (241.2) |
H | 4-F | 151--152 (72) |
64.73 64.81 |
3.34 3.37 |
17.42 17.44 |
| 2e | C14H11N3O (237.3) |
H | 4-CH3 | 143a) (70) |
-- | -- | -- |
| 2f | C17H17N3O (279.3) |
H | 4-C4H9 | 101 (69) |
73.10 72.58 |
6.13 6.19 |
15.04 15.04 |
| 2g | C14H11N3O2 (253.3) |
H | 4-OCH3 | 159--160a) (65) |
-- | -- | -- |
| 2h | C13H8ClN3O (257.7) |
H | 3-Cl | 148--149a) (71) |
-- | -- | -- |
| 2i | C13H8FN3O (241.2) |
H | 3-F | 128--129 (72) |
64.73 64.76 |
3.34 3.35 |
17.42 17.56 |
| 2j | C13H7Cl2N3O (292.1) |
H | 3,4-Cl2 | 231--232 (71) |
53.45 53.47 |
2.42 2.40 |
14.38 14.60 |
| 2k | C15H13N3O (251.3) |
H | 3,4-(CH3)2 | 158--160 (70) |
71.70 71.31 |
5.21 5.22 |
16.72 16.96 |
| 2l | C13H8ClN3O (257.7) |
Cl | H | 181--182 (47) |
60.60 60.40 |
3.13 3.19 |
16.31 16.19 |
| 2m | C13H7Cl2N3O (292.1) |
Cl | 4-Cl | 208--210 (35) |
53.45 53.41 |
2.42 2.25 |
14.38 14.25 |
| 2n | C13H7BrClN3O (336.6) |
Cl | 4-Br | 198--200 (62) |
46.39 46.49 |
2.10 2.13 |
12.48 12.39 |
| 2o | C14H10ClN3O (271.7) |
Cl | 4-CH3 | 193--195 (37) |
61.89 62.16 |
3.71 3.67 |
15.47 15.59 |
| 2p | C16H14ClN3O (299.8) |
Cl | 4-isoC3H7 | 158--159 (72) |
64.11 64.24 |
4.71 4.70 |
14.02 13.98 |
| 2r | C17H16ClN3O (313.8) |
Cl | 4-sec-C4H9 | 118.5--119.5 (53) |
65.07 65.30 |
5.14 5.15 |
13.39 13.44 |
| 2s | C13H6Cl3N3O (326.6) |
Cl | 3,4-Cl2 | 224--226 (74) |
47.81 47.65 |
1.85 1.95 |
12.87 12.78 |
|
a) Values from literature: Compound, value (oC) [ref.]:
1a, 130--131 [21];
1b, 148--149 [22];
1c, 157 [22];
1d, 131--132 [3];
1e, 150--151 [23];
1g 125--126 [23];
1h, 137--138 [22];
1j, 146--147 [3];
1k, 115--116 [24];
1l, 151--153 [25];
1m, 172--173 [26];
1n, 188--190 [27];
1o, 180 [27];
1s, 161--162 [25];
2a, 148--150 [28];
2b, 186--187 [29];
2c, 204--206 [30];
2e, 143 [31];
2g, 157 [32];
2h, 144 [32].
b) The compound 1i was mentioned in [33]. | |||||||
Table 2. 1H NMR and IR spectroscopic data
| Compd. | 1H NMR delta (ppm) |
IR |
| 1a | -- | 1 644 |
| 1b | -- | 1 637 |
| 1c | -- | 1 637 |
| 1d | -- | 1 630 |
| 1e | -- | 1 635 |
| 1f | 7.62 (dd, J = 7.69 Hz, J = 1.38 Hz, 1H, H6), 7.34--7.26 (m, 1H, H4), 7.26--7.20 (m, AA', BB', 2H, H2', H6'), 7.12--7.05 (m, AA', BB', 2H, H3', H5'), 6.96 (bs, 1H, NH), 6.76--6.67 (m, 2H, H3, H5), 2.60 (t, J = 7.55 Hz, 2H, CH2), 1.65--4.50 (m, 2H, CH2), 1.42--1.22 (m, 8H, CH2), 0.90 (t, J = 7.55 Hz, 3H, CH3) | 1 629 |
| 1g | -- | 1 635 |
| 1h | -- | 1 640 |
| 1i | 7.62 (dd, J = 8.79Hz, J = 1.79Hz, 1H, H6), 7.52--7.43 (m, 1H, H2'), 7.35--7.28 (m, 1H, H4), 7.28--7.20 (m, 1H, H6'), 7.13 (dt, J = 9.89 Hz, J = 2.20 Hz, 1H, H5'), 7.10--7.06 (m, 1H, H4'), 7.03 (bs, 1H, NH), 6.77--6.68 (m, 2H, H3, H5) | 1 637 |
| 1j | -- | 1 638 |
| 1k | -- | 1 636 |
| 1l | -- | 1 645 |
| 1m | -- | 1 636 |
| 1n | -- | 1 637 |
| 1o | -- | 1 635 |
| 1p | 10.03 (bs, 1H, NH), 7.66 (d, J = 2.47 Hz, 1H, H6), 7.62--7.57 (m, AA', BB', 2H, H2', H6'), 7.25--7.15 (m, 3H, H4, H3', H5'), 6.77 (d, J = 8.8 Hz, 1H, H3), 6.46 (bs, 2H, NH2), 2.93--2.75 (m, 1H, CH), 1.18 (d, J = 6.87, 6H, CH3) | 1 641 |
| 1r | 7.63 (d, J = 2.47 Hz, 1H, H6), 7.61--7.55 (m, AA', BB', 2H, H2', H6'), 7.21 (dd, J = 8.91 Hz, J = 2.47 Hz, 1H, H4), 7.18--7.11 (m, AA', BB', 2H, H3', H5'), 6.76 (d, J = 8.91 Hz, 1H, H3), 2.60--2.45 (m, 1H, CH), 1.60--1.45 (m, 2H, CH2), 1.16 (d, J = 7.14 Hz, 3H, CH3), 0.75 (t, J = 7.14 Hz, 3H, CH3) | 1 637 |
| 1s | -- | 1 645 |
| 1t | 7.75--7.62 (m, 3H, H6, H2', H6'), 7.26--7.12 (m, 3H, H4, H3', H5'), 6.77 (d, J = 8.79, 1H, H3) | 1 637 |
| 2a | -- | 1 683 |
| 2b | -- | 1 696 |
| 2c | -- | 1 695 |
| 2d | 8.34--8.24 (m, 2H, H5, H8), 8.16--8.10 (m, 1H, H7), 8.02--7.93 (m, 1H, H6), 7.76--7.67 (m, 2H, H2', H6'), 7.47--7.39 (m, 2H, H3', H5') | 1 695 |
| 2e | -- | 1 689 |
| 2f | 8.34--8.24 (m, 2H, H5, H8), 8.16--8.09 (m, 1H, H7), 8.01--7.94 (m, 1H, H6), 7.57--7.50 (m, AA', BB', 2H, H2', H6'), 7.42--7.32 (m, AA', BB', 2H, H3', H5'), 2.67 (t, J = 7.29 Hz, 2H, CH2), 1.68--1.54 (m, 2H, CH2), 1.42--1.26 (m, 2H, CH2), 0.92 (t, J = 7.29 Hz, 3H, CH3) | 1 690 |
| 2g | -- | 1 685 |
| 2h | 8.34--8.26 (m, 2H, H5, H8), 8.17--8.10 (m, 1H, H7), 8.02--7.95 (m, 1H, H6), 7.82--7.79 (m, 1H, H2'), 7.69--7.60 (m, 3H, H4', H5', H6') | 1 695 |
| 2i | 8.33 (dd, J = 7.81 Hz, J = 1.33 Hz, 1H, H5), 8.31--8.26 (m, 1H, H8), 8.18--8.11 (m, 1H, H7), 8.02--7.96 (m, 1H, H6), 7.69--7.51 (m, 3H, H2', H5', H6'), 7.45--7.37 (m, 1H, H4') | 1 690 |
| 2j | 8.36--8.26 (m, 2H, H5, H8), 8.19--8.11 (m, 1H, H7), 8.03 (d, J = 2.47 Hz, 1H, H2'), 8.03--7.96 (m 1H, H6), 8.79 (d, J = 8.63 Hz, 1H, H5'), 7.71 (dd, J = 8.63 Hz, J = 2.47 Hz, 1H, H6') | 1 694 |
| 2k | 8.32--8.28 (m, 1H, H5), 8.28--8.23 (m, 1H, H8), 8.16--8.08 (m, 1H, H7), 8.00--7.93 (m, 1H, H6), 7.41--7.32 (m, 3H, H2', H5', H6'), 2.31 (s, 3H, CH3), 2.29 (s, 3H, CH3) | 1 692 |
| 2l | 8.32 (d, J = 8.51 Hz, 1H, H8), 8.28 (d, J = 2.20 Hz, 1H, H5), 8.18 (dd, J = 8.51 Hz, J = 2.20 Hz, 1H, H7), 7.68--7.50 (m, 5H, H2', H3', H4', H5', H6') | 1 693 |
| 2m | 8.32 (d, J = 8.79 Hz, 1H, H8), 8.28 (d, J = 2.37 Hz, 1H, H5), 8.18 (dd, J = 8.79 Hz, J = 2.37 Hz, 1H, H7), 7.72--7.63 (m, 4H, H2', H3', H5', H6') | 1 688 |
| 2n | 8.40 (d, J = 2.20, 1H, H5), 8.18 (d, J = 8.79 Hz, 1H, H8), 7.94 (dd, J = 8.79 Hz, J = 2.20 Hz, 1H, H7), 7.73 (m, AA', BB', 2H, H3', H5'), 7.60--7.52 (m, AA', BB', 2H, H2', H6') | 1 686 |
| 2o | 8.40 (d, J = 2.20 Hz, 1H, H5), 8.17 (d, J = 8.52 Hz, 1H, H8), 7.91 (dd, J = 8.52 Hz, J = 2.20 Hz, 1H, H7), 7.54--7.48 (m, AA', BB', 2H, H2', H6'), 7.40--7.32 (m, AA', BB', 2H, H3', H5'), 2.45 (s, 3H, CH3) | 1 688 |
| 2p | 8.40 (d, J = 2.20 Hz, 1H, H5), 8.17 (d, J = 8.79 Hz, 1H, H8), 7.92 (dd, J = 8.79 Hz, J = 2.20 Hz, 1H, H7), 7.58--7.52 (m, AA', BB', 2H, H2', H6'), 7.45--7.38 (m, AA', BB', 2H, H3', H5'), 3.10--2.93 (m, 1H, CH), 1.31 (d, J = 6.87 Hz, 6H, CH3) | 1 641 |
| 2r | 8.40 (d, J = 2.37 Hz, 1H, H5), 8.17 (d, J = 8.79 Hz, 1H, H8), 7.92 (dd, J = 8.79 Hz, J = 2.37 Hz, 1H, H7), 7.59--7.51 (m, AA', BB', 2H, H2', H6'), 7.40--7.32 (m, AA', BB', 2H, H3', H5'), 2.78--2.63 (m, 1H, CH), 1.72--1.58 (m, 2H, CH2), 1.29 (d, J = 7.14 Hz, 3H, CH3), 0.88 (t, J = 7.14 Hz, 3H, CH3) | 1 688 |
| 2s | 8.32 (d, J = 8.52 Hz, 1H, H8), 8.29 (d, J = 2.37 Hz, 1H, H5), 8.18 (dd, J = 8.52 Hz, J = 2.37 Hz, 1H, H7), 8.01 (d, J = 2.47 Hz, 1H, H2'), 7.89 (d, J = 8.79 Hz, 1H, H6'), 7.68 (dd, J = 8.79 Hz, J = 2.47 Hz, 1H, H5') | 1 694 |
Antimycobacterial Activity
The antimycobacterial activity of the compounds was tested in vitro against Mycobacterium tuberculosis, M. avium, and M. kansasii, obtained from Czech National Collection of Type Cultures (CNCTC), and a clinical isolate of M. kansasii, using the micromethod for the determination of the minimum inhibitory concentration (MIC). A comparison of the MICs values for 1 unsubstituted in the acyl moiety (Table 3, [3]) and 5-chloro substituted 1 (Table 3) indicates that the introduction of chloro substituent into position 5 improved their antimycobacterial activity. 2-Amino-5-chloro-N-phenylbenzamides (1m--1t) possess a significant antimycobacterial activity against M. tuberculosis and against atypical strains, with the exception of derivative 1l (X = Cl; R = H) which was completely inactive. The most active derivative was 1r (X = Cl; R = 4-sec-C4H9). The derivative 1f (X = H; R = 4-C4H9) was inactive. The tested 3-phenyl-1,2,3-benzotriazin-4(3H)-ones (2) were generally inactive, with the exception of derivative 2e (X = H; R = 4-CH3) which was moderately active against clinical isolate of M. kansasii. From the comparison of the MICs for 3-phenyl-2H-1,3-benzoxazine-2,4(3H)-diones [1--3] and 3-phenyl-1,2,3-benzotriazin-4(3H)-ones (2), it can be concluded that the replacement of the oxygen atom by the nitrogen atom results in a decrease or even loss of the antimycobacterial activity. This is in accordance with the results obtained for 3-phenylquinazoline-2,4(1H,3H)-diones [3] and 3-phenylquinazolin-4(3H)-ones [17], but in contrast to the results for 2,3-dianilinoquinoxalines and their isosteric oxa and thia analogues [5].
Table 3. Antimycobacterial activity of the compounds expressed as MIC (micromol dm-3)
| Compd. | X | R | MIC (micromol dm-3) |
|||
| M. tbc. My 331/88 14d/21d |
M. avium My 330/88 14d/21d |
M. kansasii My 235/80 7d/14d/21d |
M. kansasii 6 509/96 7d/14d/21d |
|||
| 1h | H | 3-Cl | >250/>500 | >125/>500 | 250/500/500 | 125/250/500 |
| 1i | H | 3-F | 500/500 | 250/500 | 250/500/500 | 250/500/500 |
| 1m | Cl | 4-Cl | 31/31 | --a) | 31/31/31 | 16/31/62 |
| 1n | Cl | 4-Br | 31/31 | 31/62 | 16/31/62 | 16/31/31 |
| 1o | Cl | 4-CH3 | 62/125 | --a) | 125/>125/>250 | 31/62/125 |
| 1p | Cl | 4-isoC3H7 | 16/31 | 31/31 | 16/31/31 | 16/31/31 |
| 1r | Cl | 4-sec-C4H9 | 16/31 | 16/31 | 8/16/16 | 8/16/31 |
| 1s | Cl | 3,4-Cl2 | 32/32 | 31/31 | 8/16/31 | 8/16/31 |
| 1t | Cl | 4-F | 125/125 | >62/125 | 62/62/125 | 31/62/62 |
| 2e | H | 4-CH3 | >125/>500 | 125/>125 | --/125/>125 | --/62/125 |
| INH | 0,5/1 | >250/250 | >250/250 | 4/4 | ||
| a) not tested | ||||||
Antifungal Activity
The antifungal activity of 1 and 2 was investigated in vitro against Candida albicans, C. tropicalis, C. krusei, C. glabrata, Trichosporon beigelii, Trichophyton mentagrophytes, Aspergillus fumigatus, and Absidia corymbifera by the broth microdilution method (Table 4). A moderate antifungal activity against T. mentagrophytes was found for some 1. The most active compounds were 1c (X = H; R = 4-Br) and 1l (X = Cl; R = H). A significant activity against the other tested fungal strains was not observed. The compounds 1h, 1m--1o, 1s, 2a--2i, 2k, 2m, and 2o were completely inactive against all tested fungi. The antifungal activity of compound 2s could not be tested due to its incomplete solubility. Similarly to the antimycobacterial activity, these results show that the replacement of oxygen atom by nitrogen lead to the decrease or even loss of the antifungal activity.
Table 4. Antifungal activity of selected compounds expressed as MIC (micromol dm-3)
| Compd. | MICa) (micromol dm-3) |
|||||||
| TM 72h/120h |
CA 24h/48h |
CT 24h/48h |
CK 24h/48h |
CG 24h/48h |
TB 24h/48h |
AF 24h/48h |
AC 24h/48h |
|
| 1c | 31 62 |
1000 >1000 |
1000 >1000 |
>1000 >1000 |
1000 >1000 |
>1000 >1000 |
1000 >1000 |
>1000 >1000 |
| 1d | 125 500 |
>1000 >1000 |
>1000 >1000 |
>1000 >1000 |
>1000 >1000 |
>1000 >1000 |
>1000 >1000 |
>1000 >1000 |
| 1l | 31 62 |
500 >500 |
500 >500 |
500 >500 |
250 500 |
500 >500 |
250 >500 |
>500 >500 |
| 1t | 250 >1000 |
500 >1000 |
1000 >1000 |
1000 >1000 |
500 >1000 |
500 >1000 |
500 1000 |
1000 >1000 |
| 2l | 500 500 |
>1000 >1000 |
>1000 >1000 |
>1000 >1000 |
>1000 >1000 |
>1000 >1000 |
>1000 >1000 |
>1000 >1000 |
|
a) tested strains CA -- Candida albicans ATCC 44859 CT -- Candida tropicalis 156 CK -- Candida krusei E28 CG -- Candida glabrata 20/I TB -- Trichosporon beigelii 1188 TM -- Trichophyton mentagrophytes 445 AF -- Aspergillus fumigatus 231 AC -- Absidia corymbifera 272 | ||||||||
Photosynthesis-inhibiting Activity
Some of compounds 2 inhibited the photosynthetic electron transport in spinach chloroplasts (Table 5). This was reflected in the inhibition of oxygen evolution rate (OER). The photosynthesis-inhibiting activity of the compounds 2 expressed as the IC50 values varied in the range from 0.032 mmol dm-3 (2a; X = H; R = H) to 0.723 mmol dm-3 (2g; X = H; R = 4-OCH3). The relatively low OER-inhibiting activity of the compounds 2 is probably a consequence of their low aqueous solubility, and hence a restricted passage of the inhibitor through the hydrophilic regions of thylakoid membranes. Photosynthesis-inhibiting activity of compounds 2b, 2c, 2j, 2m, 2o, and 2r could not be determined due to their incomplete solubility. From the fact that the most active inhibitor of photochemical activity of spinach chloroplasts was the unsubstituted derivative 2a, it can be concluded that the increase in lipophilicity caused by substitution is disadvantageous for the photosynthesis-inhibiting activity of 2. These results are in accordance with those obtained for 3-phenyl-2H-1,3-benzoxazine-2,4(3H)-diones, 3-phenylquinazolin-4(3H)-ones as well as 3-phenylquinazoline-2,4(1H,3H)-diones [17]. Similar study with compounds 1 has shown that the OER-inhibiting activity of 1 is also generally low, but it exhibit a parabolic dependence on the sum of Hammett constants of X and R substituents [20].
Table 5. Inhibition of oxygen evolution rate in spinach chloroplasts by 3-phenyl-1,2,3-benzotriazin-4(3H)-ones 2 expressed as IC50 (mmol dm-3)
| Compound | X | R | IC50 (mmol dm-3) |
Compound | X | R | IC50 (mmol dm-3) |
| 2a | H | H | 0.032 | 2h | H | 3-Cl | 0.304 |
| 2d | H | 4-F | 0.232 | 2i | H | 3-F | 0.104 |
| 2e | H | 4-CH3 | 0.479 | 2k | H | 3,4-(CH3)2 | 0.393 |
| 2f | H | 4-C4H9 | 0.051 | 2p | Cl | 4-isoC3H7 | 0.104 |
| 2g | H | 4-OCH3 | 0.723 |
Experimental
General
The melting points were determined on a Kofler block and are uncorrected. The samples for elemental analyses and biological tests were dried over P2O5 at 61 oC and 66 Pa for 24 h. Elemental analyses were performed on a C,H,N,S analyzer (FISONS EA 1110, Milano). The IR spectra were measured in KBr on a Nicolet Impact 400 apparatus. The purity of the compounds was checked by TLC on precoated silica gel plates with a fluorescent indicator (Silufol UV 254 + 366, Kavalier, Votice, Czech Republic), using petroleum ether--ethyl acetate (1:1) and chloroform--acetone (9:1). Column chromatography was performed with silica gel 60 (0.040--0.063 mm, Merck, Darmstadt, Germany) and mixtures of petroleum ether with ethyl acetate. The 1H NMR spectra of new compounds were recorded for DMSO solutions at ambient temperature on a Varian Mercury--Vx BB 300 spectrometer operating at 300 MHz. Chemical shifts were recorded as delta values in parts per million (ppm), and were indirectly referenced to tetramethylsilane via the solvent signal (2.49 for 1H). Multiplicities are given together with the coupling constants (in Hz).
2-Amino-N-phenylbenzamides 1a--1k
A suspension of 2H-3,1-benzoxazine-2,4(1H)-dione (20 mmol) in glacial acetic acid (150 ml) was added gradually to a stirred solution of appropriate aniline (40 mmol) in the same solvent (50 ml). The mixture was slowly heated to boil with stirring, and then poured into water (1 dm-3). After 24 h, the product was filtered off and crystallized from aqueous ethanol.
2-Amino-5-chloro-N-phenylbenzamides 1l--1t
6-Chloro-2H-3,1-benzoxazine-2,4(1H)-dione (2.5 mmol) was melted with the appropriate aniline (2.5 mmol) for 10 minutes (170--180 oC). After cooling to room temperature, the mixture was separated by column chromatography on 10 g of silica gel, using mixtures of petroleum ether with ethyl acetate. The product was eluated by petroleum ether--ethyl acetate (90:10), and then recrystallized from aqueous ethanol.
1,2,3 Benzotriazin-4(3H)-ones 2a--2s
2-Amino-N-phenylbenzamide 1a--1s (5 mmol) was added to a mixture of n-propanol (8 ml) and H2SO4 (1,5M; 8 ml). To a stirred ice-cold mixture was added 30% aqueous sodium nitrite (5 mmol) dropwise. The precipitate was filtered off, washed with water, and recrystallized from aqueous ethanol.
Biological assays
Antimycobacterial activity
For the in vitro evaluation of antimycobacterial activity of the substances, the following strains were used: Mycobacterium tuberculosis CNCTC My 331/88, M. kansasii CNCTC My 235/80, and M. avium CNCTC My 330/88, obtained from the Czech National Collection of Type Cultures (CNCTC), National Institute of Public Health, Prague, and a clinic isolate of M. kansasii 6509/96. Antimycobacterial activity of the compounds against these strains was determined in the Sula semisynthetic medium (SEVAC, Prague). Each strain was simultaneously inoculated into a Petri dish containing the L�wenstein-Jensen medium for the control of the sterility of the inoculum and its growth. The compounds were added to the medium in a dimethyl sulfoxide (DMSO) solutions. The following concentrations were used: 1 000, 500, 250, 125, 62, 31, 16, 8, and 4 micromoldm-3. The minimum inhibitory concentrations (MICs) were determined after incubation at 37 oC for 7 (only M. kansasii), 14 and 21 days. MIC was the lowest concentration of a substance, at which the inhibition of the growth occurred. The compound is considered as active, when its MIC is lower than 1000 micromol dm-3. Isoniazid was used as the standard.
Antifungal activity
Followed fungal organisms were selected for in vitro antifungal susceptibility testing by the broth microdilution method [34]: Candida albicans ATCC 44859, C. tropicalis 156, C. krusei E28, C. glabrata 20/I, Trichosporon beigelii 1188, Trichophyton mentagrophytes 445, Aspergillus fumigatus 231, and Absidia corymbifera 272. Prior to testing, each strain was passaged onto SDA. Fungal inocula were prepared by suspending yeast or conidia in sterile water to obtain a final inoculum of 5.0 +- 0.2 x 103 cfu cm-3. Antifungal activity of the compounds in vitro was determined in tissue culture medium RPMI 1640 (SEVAC) buffered to pH 7.0 with 0.165M morpholinepropanesulfonic acid (Sigma). Each substance was dissolved in DMSO. The concentrations tested were 1000, 500, 250, 125, 62, 31, 16, 8, 4, and 2 micromol dm-3 provided a given compound was soluble in DMSO and did not precipitate in RPMI. Drug-free controls were included. The minimum inhibitory concentrations (MIC) were determined after 24 and 48 h of static incubation at 35 oC. In the case of T. mentagrophytes, the MICs were recorded after 72 and 120 h of incubation.
Study of oxygen evolution rate in spinach chloroplasts
Chloroplasts were prepared by the procedure described by Walker [35]. The inhibitory activity of compounds 2 concerning oxygen evolution rate (OER) in spinach chloroplasts was investigated spectrophotometrically (Specord UV VIS, Zeiss, Jena) in the presence of the electron acceptor 2,6-dichlorophenol-indophenol (DCPIP) according to Kralova et al. [36] and the rate of photosynthetic electron transport was monitored as a photoreduction of DCPIP. The chlorophyll (Chl) content was 30 mg dm-3. Samples were irradiated from the distance of 1 dm with a halogen lamp (250 W) through a 4-cm water filter to prevent overheating of the samples. The activity of compounds 2 was expressed as IC50 values, i. e. molar concentration causing a 50% decrease of OER with respect to the untreated control. For low solubility of the studied compounds in water, these were dissolved in DMSO. The applied solvent content (up to 4 v/v. %) did not affect the photochemical activity in spinach chloroplasts.
Acknowledgements: The authors wish to thank Mrs. J. Zizkova from the Department of Inorganic and Organic Chemistry for measurements of the IR spectra and Mrs. D. Karlickova from the Department of Pharmaceutical Chemistry and Drug Control, Faculty of Pharmacy, Charles University, for elemental analyses. Thanks are due to Dr. D. Mikulasova from the Department of Biochemistry, Faculty of Natural Sciences, Comenius University, Bratislava, for her assistance in the preparation of chloroplast, and to Mr. T. Vojtisek from the Department of Pharmaceutical Chemistry and Drug Control, Faculty of Pharmacy, Charles University, for transformation of the poster into html format. This study was supported by the Ministry of Education of the Czech Republic (Grant No. 1301/2000, project No. VS97124, research projects No. MSM 111600001 and No. MSM 111600002), by the Grant Agency of the Czech Republic (grant No. 203/99/0030), and by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic (grant No. 1/7262/20).
References
[1] Waisser K., Kubicova L., Klimesova V., Odlerova Z.: Collect. Czech. Chem. Commun. 1993, 58, 2977.
[2] Waisser K., Hladuvkova J., Kubicova L., Klimesova V., Buchta V., Odlerova Z.: Sci. Pharm. 1996, 64, 701.
[3] Waisser K., Machacek M., Dostal H., Gregor J., Kubicova L., Klimesova V., Kunes J., Palat K. Jr., Hladuvkova J., Kaustova J., Moellmann U.: Collect. Czech. Chem. Commun. 1999, 64, 1902.
[4] Waisser K., Hladuvkova J., Gregor J., Rada T., Kubicova L., Klimesova V., Kaustova J.: Arch. Pharm. Pharm. Med. Chem. 1998, 331, 3.
[5] Waisser K., Beckert R., Slosarek M., Janota J.: Pharmazie 1997, 52, 797.
[6] Satzinger G.: Ger. 1 249 876, 1967.
[7] Kornet M. J.: J. Heterocycl. Chem. 1997, 34, 1391.
[8] Farbenfabriken Bayer A.-G.: Ger. 1 121 055, 1962.
[9] Satzinger G., Herrmann M.: Ger. Offen. 2 012 094, 1971.
[10] Bayer A.-G.: Ger. Offen. DE 3241265, 1984.
[11] Kralova K., Sersen F., Miletin M., Hartl J. : Chem. Papers 1998, 52, 52.
[12] Kralova K., Sersen F, Gasparova R., Lacova M.: Chem. Papers 1998, 52, 776.
[13] Sidoova E., Kralova K., Loos D.: Molecules 1998, 3, 135.
[14] Sidoova E., Kralova K., Loos D.: Molecules 1999, 4, 73.
[15] Miletin M., Hartl J., Dolezal M., Odlerova Z., Kralova K, Machacek M: Molecules 2000, 5, 208.
[16] Dolezal M., Hartl J., Miletin M., Machacek M., Kralova K.: Chem. Papers 1999, 53, 126.
[17] Kubicova L., Kudelova P., Dostal H., Waisser K.: Folia Pharm. Univ. Carol. 2000, 25, 81.
[18] Goldberg A. A., Jefferies H. S., Turner H. S.: Quart. J. Pharm. 1948, 21, 10.
[19] Kubicova L., Waisser K.: Cesk. Slov. Farm. 1997, 46, 99.
[20] Kubicova L., Kralova K., Dostal H., Sersen F., Machacek M., Waisser K.: Folia Pharm. Univ. Carol. 2000, 25, 73.
[21] Wagner G., Rothe L.: Pharmazie 1969, 24, 513.
[22] Grammaticakis P.: Bull. Soc. Chim. Fr. 1962, 490.
[23] Clark R., Wagner E. C.: J. Org. Chem. 1944, 9, 55.
[24] Clark C. R., Lin Ch.-M., Sansom R. T.: J. Med. Chem. 1986, 29, 1534.
[25] Jacobs R. L.: J. Heterocycl. Chem. 1970, 7, 1337.
[26] Kozhevnikov Ju. V., Petjunin P. A.: Khim. Geterotsikl. Soedin. 1969, 747.
[27] Konshin M. E.: Khim. Geterotsikl. Soedin. 1970, 974.
[28] Ludwig M., Bauerova I.: Collect. Czech. Chem. Commun. 1998, 63, 2075.
[29] Barker A. J., Peterson T. McC., Smalley R. K., Suschitzky H.: J. Chem. Soc. Perkin. Trans. 1979, 1, 2203.
[30] Kerber R. C.: J. Org. Chem. 1972, 37, 1587.
[31] Mehner J.: J. Prakt. Chem. 1901, 63, 241.
[32] Grammaticakis P.: C. R. Hebd. Seances Acad. Sci. 1956, 243, 2094.
[33] Yu M. J., Thrasher K. J., McCowan J. R., Mason N. R., Mendelsohn L. G.: J. Med. Chem. 1991, 34, 1505.
[34] Klimesova V., Svoboda M., Waisser K., Machacek M., Buchta V., Odlerova Z.: Arch. Pharm. Pharm. Med. Chem. 1996, 329, 438.
[35] Walker D. A.: Preparation of higher plant chloroplasts. In: Methods in Enzymology, Vol. 69, Part C, pp. 94--104, San Pietro A. (ed.), Academic Press, New York, London, Toronto, Sydney, San Francisco 1980.
[36] Kralova K., Sersen F., Sidoova E.: Chem. Papers 1992, 46, 348.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with C0015 as the message subject of your e-mail.