EXPERIMENTAL SECTION
[C0032]
Huan-Ming Chen and Ramachandra S. Hosmane*
Laboratory for Drug Design and Synthesis
Department of Chemistry & Biochemistry
University of Maryland, Baltimore County
1000 Hilltop Circle, Baltimore, Maryland 21250, USA
* Phone: 410-455-2520 Fax: 410-455-1148 E-Mail: [email protected]
Received: 14 August 2000 / Uploaded: 17 August
Key Words: Synthesis, Nucleoside Analogue, Imidazo[4,5-d]pyridazine, 2'-O-Methyl Nucleoside
ABSTRACT
Synthesis of the title compound,1-(2'-O-methyl-ß-D-ribofuranosyl)-1H-imidazo-[4,5-d]pyridazine-
-4,7(5H,6H)-dione (1), is reported. It was synthesized in four steps,
starting from Methyl 1-(ß-D-ribofuranosyl)imidazo-4,5-dicarboxylate
(2). The 3',5'-hydroxyl groups of 2 was protected with a
bis-silylating agent to form 3, which was then methylated to form
the corresponding 2'-O-methyl derivative 5. The silyl deprotection
of the latter (to form 6), followed by treatment with hydrazine
afforded the target nucleoside 1. The reported nucleoside has potentially
beneficial applications in biomedicine based on antisense and triple-helical
nucleic acid technologies.
INTRODUCTION
2'-O-Methyl ribonucleotides are gaining wide attention
in recent years because of their newly discovered, diverse biomedical applications
in viral and cancer therapies.1,2 Oligo-2'-O-methyl-ribonucleotides
(2'-O-methyl RNA) were recently found to strongly inhibit restriction
endonuclease via formation of triple-helices with oligoribonucleotides
(RNA) and genomic sequences containing the recognition site for the class
II-S restriction enzyme, Ksp632-I.1 Synthetic 2'-O-methyl-modified
hammerhead ribozymes, designed to be complementary to the RNA component
of human telomerase, was shown to exhibit dose-dependent inhibition of
human telomerase activity in tissue culture systems with a 0.4 micromolar
IC50 value.2 It has also been demonstrated that specific
2'-O-methyl-oligoribonucleotides, but not the corresponding 2'-deoxyribo
nucleotides, bind to E.coli tRNA (Cys), and inhibit aminoacylation
of the latter by cysteine tRNA synthetase.3 Furthermore, because
of the significantly enhanced stability of their hybrids with complementary
RNA as compared to that of the corresponding DNA.RNA duplexes, 2'-O-methyl-oligoribonucleotides
are an attractive class of compounds for antisense-based therapeutic applications.4,5
We report here the synthesis of a nucleoside analogue containing a methoxy
functionality at the 2'-position, namely,1-(2'-O-methyl-ß-D-ribofuranosyl)-1H-imidazo[4,5-d]pyridazine-4,7(5H,6H)
-dione (1). The latter can be considered an analogue of purine nucleoside
in which a pyridazine moiety replaces a pyrimidine in fusion to an imidazole
ring. Our molecular modeling studies suggest that nucleoside 1 has
some unique structural features that would make it an attractive building
block for oligonucleotides for potential antisense and triple-helical applications.
These features include, but are not limited to, its potential capability
to base-pair with cytidine, like guanosine, forming three H-bonds, but
with a significantly shortened sugar-sugar (C-1' to C-1') distance, which
in turn might lead to the decreased interstrand reach and consequently
somewhat compressed double-helix. The study of effect of such a compression
on the double-helical or triple-helical conformations, interactions, and
stability would be interesting and rewarding.
RESULTS & DISCUSSION
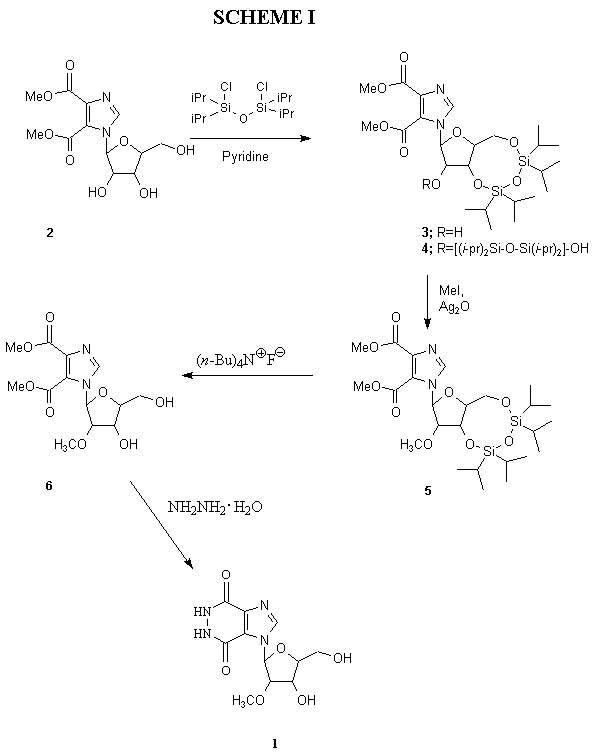
Synthesis of the target compound commenced with methyl
1-(ß-D-ribofuranosyl)imidazole-4,5-dicarbo- xylate (2)6
(Scheme I), which was selectively protected at the 3',5'-position
using the Markiewicz reagent7 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane
to form the corresponding TIPDS derivative 3 as the major product,
along with a small amount of the completely silyl-protected derivative
4.
Methylation of 3 with methyl iodide in the presence of silver oxide
afforded the corresponding 2'-O-methyl derivative 5. The
silyl deprotection of the latter with tetra-n-butylammonium fluoride
gave the free nucleoside 6. The ring-closure of 6 to form
the target 1 was accomplished by treatment with hydrazine. The synthesis
of the parent ribofuranoside of 1, containing a 2'-OH group in place
of 2'-OMe, has long been reported in the literature, both by imidazole
ring-closure6f as reported here, as well as by glycosylation
of the parent heterocyclic base, imidazo[4,5-d]pyridazine-4,7(5H,6H)-dione.6b

EXPERIMENTAL SECTION
1H NMR spectra were recorded on a General Electric
QE-300 (300 MHz) instrument. The spectral data are reported in the following
format: chemical shift (all relative to Me4Si as an internal
reference standard unless otherwise indicated), multiplicity (s = singlet,
d = doublet, dd = doublet of doublets, t = triplet, q = quartet, m = multiplet,
b = broad ), integration, coupling constants, exchangeability after D2O
addition, and assignment of resonances. Elemental Microanalyses were performed
by Atlantic Microlab, Inc., Norcross, Georgia. The mass spectra were recorded
at the Mass Spectral Facility, Department of Biochemistry, Michigan State
University. Thin layer chromatography was performed on Merck Kieselgel
60 GF254 plates ( 0.2 mm thickness). Melting points were determined
on a Thomas-Hoover capillary melting point apparatus, and are uncorrected.
Methyl 1-[(3',5'-O-(1,1,3,3,-tetraisopropyldisiloxan-1,3-diyl))-ß-D-ribofuranosyl]-4,5-imidazoledi- carboxylate (3) and Methyl1-[((2'-O-(3-hydroxy-1,1,3,3,-tetraisopropyldisiloxyl)-3',5'-O-(1,1,3,3, -tetraisopropyldisiloxan-1,3-diyl))-ß-D-ribofuranosyl]-4,5-imidazoledicarboxylate (4)
To a solution of dry methyl 1-ß-D-ribofuranosyl-4,5-imidazoledicarbo xylate6 (2) (500 mg, 1.58 mmol) in dry pyridine (10 mL) was added 1,3-dichloro-1,1,3,3-tetraisopropyl disiloxane7 (550 mg, 1.75 mmol), and the mixture was stirred for 4 h at ambient temperature with protection from moisture. Volatile materials were evaporated in vacuo, and the residue was dissolved in chloroform. The chloroform solution was washed twice with cold water and dried over anhydrous sodium sulfate. The residue after evaporation was purified by silica gel flash chromatography, eluting with chloroform to give 3 and 4 as a colorless liquids, whose yield, spectral and analytical data are given below.
Compound 3: Yield 800 mg (91%), Rf 0.38 (chloroform/ methanol, 30:1); 1H-NMR (CDCl3) 8.10 (s, 1H, imidazole), 6.11 (s, 1H, 1'-H), 4.44 (dd, 1H, J=4.2 and 9.0 Hz, 3'-H), 4.26 (d, 1H, Jgem=13.5 Hz, 5'-H), 4.18 (d, 1H, J=4.2 Hz, 2'-H), 4.16 (dd, 1H, J=9.0 and 2.4 Hz, 4'-H), 4.03 (dd, 1H, Jgem=13.5 Hz, J5',4'=2.4 Hz, 5'-H), 3.95 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 2.89 (brs, 1H, 2'-OH, exchangeable with D2O), 1.05 (m, 28H, isopropyl groups); 13C-NMR (CDCl3, 75.48 MHz) 12.45 (CHSi), 12.82 (CHSi), 12.90 (CHSi), 13.35 (CHSi), 16.81 (CCH3), 16.87 (CCH3), 16.97 (CCH3), 16.97 (CCH3), 17.26 (CCH3), 17.26 (CCH3), 17.32 (CCH3), 17.42 (CCH3), 52.50 (OCH3), 52.78 (OCH3), 59.91 (C-5'), 68.43 (C-3'), 76.82 (C-2'), 81.87 (C-4'), 91.54 (C-1'), 122.83 (C-4 or 5), 137.26 (C-2), 138.57 (C-5 or 4), 160.26 (C=O), 162.91 (C=O).
Anal. Calcd. for C24H42N2O9Si2 (MW 558.78): C, 51.59; H, 7.58; N, 5.01. Found: C, 51.50; H, 7.57; N, 5.04.
Compound 4: Yield 120 mg (9%), Rf 0.45 (chloroform/ methanol, 30:1); 1H-NMR (CDCl3) 8.37 (s, 1H, imidazole), 6.06 (s, 1H, 1'-H), 4.48 (d, 1H, J=3.3 Hz, 2'-H), 4.35 (dd, 1H, J=3.3 and 9.6 Hz, 3'-H), 4.31 (d, 1H, Jgem=13.8 Hz, 5'-H), 4.27 (dd, 1H, J=9.6 and 2.4 Hz, 4'-H), 4.02 (dd, 1H, Jgem=13.8 Hz, J5',4'=2.4 Hz, 5'-H), 3.93 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 1.03 (m, 56H, isopropyl groups); 13C-NMR (CDCl3, 75.48 MHz) 12.51 (CHSi), 12.91 (CHSi), 13.05 (CHSi), 13.05 (CHSi), 13.24 (CHSi), 13.35 (CHSi), 13.39 (CHSi), 13.48 (CHSi), 16.59 (CCH3), 16.78 (CCH3), 16.81 (CCH3), 16.81 (CCH3), 17.06 (CCH3), 17.06 (CCH3), 17.06 (CCH3), 17.23 (CCH3), 17.26 (CCH3), 17.26 (CCH3), 17.30 (CCH3), 17.37 (CCH3), 17.42 (CCH3), 17.51 (CCH3), 17.55 (CCH3), 17.65 (CCH3), 52.56 (OCH3), 53.17 (OCH3), 59.56 (C-5'), 68.47 (C-3'), 77.95 (C-2'), 81.44 (C-4'), 92.15 (C-1'), 121.16 (C-4 or 5), 138.64 (C-2), 139.48 (C-5 or 4), 161.49 (C=O), 163.11 (C=O).
Anal. Calcd. for C36H70N2O11Si4
(MW 819.30): C, 52.78; H, 8.61; N, 3.42. Found: C, 52.45; H, 8.89; N, 2.93.
Methyl 1-[(2'-O-Methyl-3',5'-O-(1,1,3,3,-tetraisopropyldisiloxan-1,3-diyl))-ß-D-ribofuranosyl] -4,5-imidazoledicarboxylate (5)
A mixture of methyl 1-[(3',5'-O-(1,1,3,3,-tetraisopropyldisiloxan-1,3-diyl))-ß-D-ribofuranosyl]-4,5- imidazoledicarboxylate (3) (560 mg, 1 mmol), Ag2O (1.85 g, 8 mmol) and MeI (10 mL) was refluxed for 5 h. The mixture was diluted with Et2O, and was filtered over Celite.TM The filtrate was concentrated in vacuo and the residue was purified by silica gel column chromatography, eluting with chloroform, to give 5 as a colorless oily product in quantitative yield, Rf 0.35 (hexane/ethyl acetate, 3:1); 1H-NMR (CDCl3) 8.20 (s, 1H, imidazole), 6.02(s, 1H, 1'-H), 4.42 (dd, 1H, J=4.2 and 9.6 Hz, 3'-H), 4.28 (d, 1H, Jgem=13.8 Hz, 5'-H), 4.16 (dd, 1H, J=9.6 and 2.4 Hz, 4'-H), 4.00 (dd, 1H, Jgem=13.8 Hz, J5',4'=2.4 Hz, 5'-H), 3.94 (s, 3H, COOCH3), 3.93 (s, 3H, COOCH3), 3.78 (d, 1H, J=4.2 Hz, 2'-H), 3.69 (s, 3H, OCH3), 1.05 (m, 28H, isopropyl groups); 13C-NMR (CDCl3, 75.48 MHz) 12.53 (CHSi), 12.90 (CHSi), 12.96 (CHSi), 13.46 (CHSi), 16.87 (CCH3), 17.01 (CCH3), 17.01 (CCH3), 17.15 (CCH3), 17.30 (CCH3), 17.30 (CCH3), 17.38 (CCH3), 17.49 (CCH3), 52.45 (COOCH3), 52.62 (COOCH3), 59.46 (OCH3), 59.96 (C-5'), 68.66 (C-3'), 81.59 (C-4'), 84.99 (C-2'), 90.76 (C-1'), 122.84 (C-4 or 5), 137.55 (C-2), 138.57 (C-5 or 4), 160.23 (C=O), 163.06 (C=O).
Anal. Calcd. for C25H44N2O9Si2
(MW 572.80): C, 52.42; H, 7.74; N, 4.89. Found: C, 52.46; H, 7.85; N, 4.63.
Methyl 1-(2'-O-Methyl-ß-D-ribofuranosyl)-4,5-imidazoledicarboxylate (6)
A 1M solution of tetra-n-butylammonium fluoride in THF (2 mL, 2 mmol) was added to an ice-cooled solution of methyl 1-[(2'-O-Methyl-3',5'-O-(1,1,3,3,-tetraisopropyl disiloxan-1,3-diyl))--D-ribo furanosyl]-4,5-imidazoledicarboxylate (5) (573 mg, 1 mmol) in 10 mL of dry THF. The reaction mixture was stirred for 45 min at 0 oC. The solvent was evaporated in vacuo and the pure product was obtained as a foam after silica gel column chromatography, elting with a mixture of chloroform-methanol (20:1), 85 % yield, Rf 0.29 (chloroform/methanol, 10:1); 1H-NMR (CDCl3) 8.67 (s, 1H, imidazole), 6.17 (d, 1H, J=2.1Hz, 1'-H), 5.03 (brs, 1H, 3'-OH, exchangeable with D2O), 4.47 (t, 1H, 5'-OH, exchangeable with D2O), 4.12 (m, 2H, 2',3'-H), 3.93 (s, 3H, COOCH3), 3.91 (s, 3H, COOCH3), 3.91 (m, 2H, 4',5'-H), 3.60 (s, 3H, OCH3), 3.32 (m, 1H, 5'-H); 13C-NMR (CDCl3, 75.48 MHz) 52.31 (COOCH3), 52.74 (COOCH3), 59.10 (OCH3), 60.03 (C-5'), 68.15 (C-3'), 84.85 (C-4'), 85.58 (C-2'), 88.83 (C-1'), 123.96 (C-4 or 5), 136.43 (C-5 or 4), 138.37 (C-2), 160.52 (C=O), 162.26 (C=O).
Anal. Calcd. for C13H18N2O8
(MW 330.29): C, 47.27; H, 5.49; N, 8.48. Found: C, 47.54; H, 5.64; N, 8.28.
1-(2'-O-Methyl-ß-D-ribofuranosyl)-1H-imidazo[4,5-d]pyridazine-4,7(5H,6H)-dione
(1)
ACKNOWLEDGMENT
This research was supported by a grant from the National Institutes of Health (#RO1 CA 71079). The Michigan State University Mass Spectrometry Facility was supported in part from a grant (# P41RR00480-0053) from the National Institutes of Health
REFERENCES
1. Ushijima, K.; Ishibashi, T.; Yamakawa, H.; Tsukahara, S.; Takai, K.; Maruyama, T.; Takaku, H. Biochemistry 1999, 38, 6570.
2. Wan, M. S.; Fell, P. L.; Akhtar, S. Antisense Nucleic Acid Drug Dev. 1998, 8, 309.
3. Hou, Y. M.; Gamper, H. B. Biochemistry 1996, 35, 15340.
4. Lubini, P.; Zurcher, W.; Egli, M. Chem. Biol.1994, 1, 39.
5. Lesnik, E. A.; Guinosso, C. J.; Kawasaki, A. M.; Sasmor, H.; Zounes, M.; Cummins, L. L.; Ecker, D. J.; Cook, P. D.; Freier, S. M. Biochemistry1993, 32, 7832.
6. (a) Wyss, P. C.; Fischer, U. Helv. Chim. Acta1978, 61, 3149. (b) Cook, P. D.; Dea, P.; Robins, R. K. J. Heterocyclic Chem.1978, 15, 1. (c) Cook, P. D.; Robins, R. K. in Nucleic Acid Chemistry, Part 1, Townsend, L. B and Tipson, R. S., Ed.; John Wiley, Inc., New York, 1978, p 211. (d) Fischer, U.; Wyss, P. C. Ger. Offen. 2735458, 1978; Chem. Abstr. 89:24726e, 1978. (e) Fischer, U.; Wyss, P. C. Belg. 857512, 1978; Chem. Abstr. 89:44128q, 1978. (f) Tapiero, C.; Imbach, J. L.; Panzicka, R. P.; Townsend, L. B. J. Carbohyd. Nucleosides Nucleotides1976, 3, 191.
7. Markiewicz, Wojciech T. J. Chem. Res. (S) 1979, 1, 24.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with C0032 as the message subject of your e-mail.