A Direct Synthesis of Pyrrolidines Including Nornicotine
Miguel Yus,* Tatiana Soler and Francisco Foubelo
Departamento de Química Orgánica, Universidad de Alicante, E-03080 Alicante,
Spain. Tel. +34 6 5903548, Fax +34 6 5903549, E-mail: [email protected]
http://www.ua.es/dept.quimorg/
Received: 20 August 2001 / Uploaded 21 August 2001
Keywords: Lithiation, imines, pyrrolidines.
Introduction
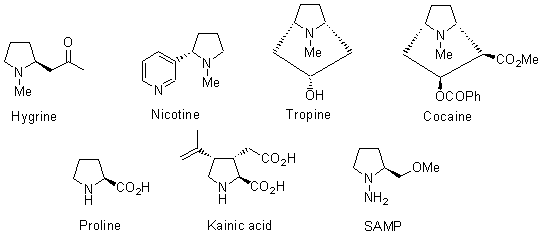
Pyrrolidines are important structural units because they take part in many natural occurring alkaloids, such as hygrine, nicotine, tropine or cocaine, which show strong biological activity.1,2 Other compounds having this moiety belong to the amino acids kingdom, such as proline or kainic acid. In addition, prolinol derivatives, such as the couple SAMP/RAMP have found important applications as chiral auxiliaries, mainly in stereoselective deprotonation of hydrazones.3

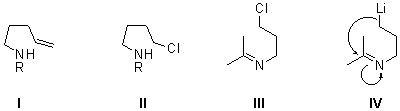
Methodologies to construct the pyrrolidine ring include inter- or intramolecular reactions.4-6 To the first group belong: (a) radical, electrophilic or metal-mediated metathesis of diallyl amines; (b) 1,3-dipolar cycloaddition starting from aziridines; (c) samarium diiodide-promoted cyclization of bis(?-ketoalkyl)amine derivatives; and (e) aminomercuration of 1,5-hexadiene.7 Among procedures involving intramolecular reactions, the most useful methods start from bishomoallyl amines (I), or 4-chloroalkyl amines (II) by radical (or electrophilic) cyclization, or an SN reaction, respectively.5 In this paper we propose a new approach to synthesize the pyrrolidine ring starting from chloroimines III (easily available from the corresponding carbonyl compounds and 3-chloropropylamine) and performing a chlorine-lithium exchange using a 4,4-di-tert-butylbiphenyl (DTBB) catalyzed lithiation,8 so the corresponding functionalized intermediate9 of type IV can undergo intramolecular addition to the imine group giving the expected cyclization reaction.
Results and Discussion
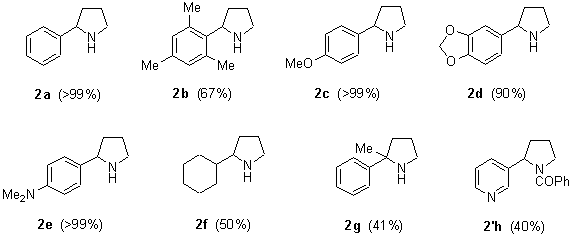
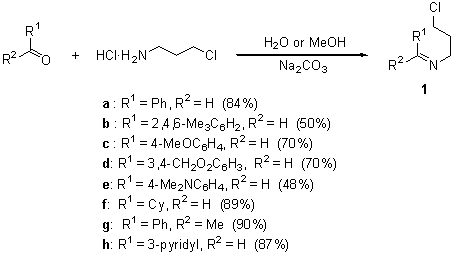
The reaction of different N-(3-chloropropyl) imines 1a-h with an excess of lithium powder (1:10 molar ratio) and a catalytic amount of DTBB (1:0.1 molar ratio; 5% molar) in THF at –78şC for 2 h led, after hydrolysis with water, to the expected pyrrolidines 2a-h (Scheme 1 and Chart 1). In the case of nornicotine (2h), we had problems for its isolation and purification due to its high solubility in water and easy decomposition by column chromatography. For these reasons, we transformed the initially formed nornicotine (2h) into its N-benzoyl derivative (2’h), which was isolated in pure form.
Scheme 1
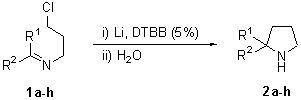
Chart 1
From a mechanistic point of view, we think that a chlorine-lithium exchange takes place (without changing the imine functionality) giving an intermediate of type IV, in which an intramolecular addition occurs generating the corresponding cyclization to yield the expected N-lithium pyrrolidines. Final lithium-hydrogen exchange affords pyrrolidines 2.
Starting chloroimines 1 were easily prepared by reaction of commercially available 3-chloropropylamine hydrochloride with the corresponding carbonyl compound (R1R2CO) and sodium carbonate in water (for R1 ? H, R2 = H; compounds 1a-f,h) or methanol (R1,R2 ? H; compound 1g) at room temperature (Scheme 2).10
Scheme 2
Conclusion
In summary, the methodology reported here represents a new route to prepare substituted pyrrolidines, including nornicotine, starting from very simple materials, such as carbonyl compounds (mainly aldehydes) and 3-chloropropylamine, this being a new disconnection to generate this type of biologically interesting units (Scheme 3).
Scheme 3
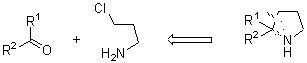
Acknowledgements
The DGICYT of the Spanish Ministerio de Educación y Cultura (no. PB97-0133) and generously supported the work presented here. I. G. thanks MEC for a predoctoral grant.
References and Notes
[2] For biosynthesis studies, see: (a) Terssell, K. B. G. Natural Product Chemistry, 2nd Edn.; Apotekarsocieteten: Stokholm, 1977; chapter 8. (b) Misra, N.; Luthra, R.; Singh, K. L.; Kumar, S. In Comprehensive Natural Products Chemistry; Barton, D.; Nakanishi, K; Meth-Cohn, O.; Kelly, I. W., Eds.; Elsevier: Amsterdam, 1999; vol 4, chapter 4.03.
[3] Enders, D. In Asymmetric Synthesis; Morrison, J. D.; Ed.; Academic Press: Orlando, 1984; vol 3, chapter 4, pp 275-339.
[4] For general information, see: Mitchinson, A.; Nadin, A. J. Chem. Soc., Perkin Trans. 1 2000, 2862-2892 (Contemporary review) and former reviews on "Saturated nitrogen heterocycles" cited therein.
[5] For a review, see: Pichon, M.; Figadčre, B. Tetrahedron: Asymmetry 1996, 7, 927-964.
[6] Pyrrolidine is prepare industrially following two procedures: (a) Hydrogenation of pyrrol using a Rh-Al catalyst, and (b) reaction of 1,4-butanediol with ammonia at 350şC in the presence of aluminium oxide. See, for instance Acheson, R. M. An Introduction to the Chemistry of Heterocyclic Compounds; John Wiley & Sons: New York, 1976; chapter III.
[7] (a) Gómez-Aranda, V.; Barluenga, J.; Yus, M. An. Quim. 1972, 68, 221-222. (b) Barluenga, J.; Nájera, C.; Yus, M. J. Heterocyclic Chem. 1981, 18,1297-1299.
[8] Reviews: (a) Yus, M. Chem. Soc. Rev. 1996, 155-161. (b) Ramón, D. J.; Yus, M. Eur. J. Org. Chem. 2000, 225-237 (Microreview).
[9] Reviews: (a) Nájera, C.; Yus, M. Trends Org. Chem. 1991, 1, 155-181. (b) Nájera, C.; Yus, M. Recent Res. Dev. Org. Chem. 1997, 1, 67-96. (c) Yus, M.; Foubelo, F. Rev. Heteroatom. Chem. 1997, 17, 73-107.
[10] Grigg, R.; Nimal Gunaratne, H. Q.; Kemp, J. J. Chem. Soc., Perkin
Trans. 1 1984, 41-46.
Miguel Yus was born in Zaragoza (Spain) in 1947, and received his BSc (1969), MSc (1971) and PhD (1973) degrees from the University of Zaragoza. After spending two years as a postdoctoral fellow at the Max Planck Institut für Kohlenforschung in Mülheim a.d. Ruhr he returned to Spain to the University of Oviedo where he became assistant professor in 1977, being promoted to full professor in 1987 at the same university. In 1988 he moved to a chair in Organic Chemistry at the University of Alicante where he is currently the head of the Organic Chemistry Department. Professor Yus has been visiting professor at different institutions and universities such as ETH-Zentrum, Oxford, Harvard, Uppsala, Marseille, Tucson, Okayama and Paris(VI). He is co-author of about 275 papers mainly in the field of development of new methodologies involving organometallic intermediates. His current research interest is focused on the preparation of very reactive functionalised organometallic compounds and their use in synthetic organic chemistry, arene-catalysed activation of different metals and preparation of new metal-based catalysts for homogeneous and heterogeneous selective reactions.
Tatiana Soler was born in Alicante (Spain) in 1974, and received her BSc (1997), MSc (1998) and PhD (2001) degrees from the University of Alicante. During this time she was working in the structural modification of carbohydrates and steroids through functionalyzed organolithium intermediates. In 1998, she was awarded the VII San Alberto Magno Prize for the best Master Thesis.
Francisco Foubelo was born in Carreńa-Cabrales (Spain) in 1961, and received
his BSc (1984), MSc (1986) and PhD (1989) degrees from the University of Oviedo.
After spending two years as a postdoctoral fellow at Princeton University (NJ,
USA), he returned to Spain to the University of Alicante where he became
associate professor in 1995. His current research interests include the
preparation of new chiral polyfunctionalized anionic synthons and the
development of new methodologies for preparing organolithium compounds.