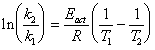 (1)
(1) MICROWAVE ASSISTED HETEROGENEOUS AND HOMOGENEOUS REACTIONS
Farid CHEMAT (1) and Erik ESVELD (2)
(1) Laboratoire de Chimie des Substances Naturelles et des
Sciences des Aliments Faculté des Sciences et Technologies, Université de la
Réunion 15 avenue René Cassin, B.P. 7151, F-97715 Saint Denis messag cedex
9
La Réunion, France D.O.M. E-mail: [email protected]
(2) Department of Process Technology, Agrotechnological Research
Institute ATO-DLO, P.O. Box 17, NL- 6700 AA Wageningen, The Netherlands. E-mail:
[email protected]
Received: 15 August 2001 / Uploaded 22 August 2001
I. INTRODUCTION
Nowadays, the important development in the industry is that many traditional chemical processing techniques are reaching their optimum performance while consumers demand’s stretch and governmental regulations tighten. The central starting point is the knowledge of chemical processes, which cause activation of the reaction or deterioration of the quality of the products. With this knowledge and by combining several techniques in an intelligent way it is possible to accelerate processes and to reduce the chemical uses. Microwave technology can be very useful for chemical processing, because products are heated directly instead of by convection and conduction. This means a reduction of the total processing time, no overheating and degradation of the product, and preservation of the product quality.
The purpose of the study is to investigate the microwave superheating phenomena and their impact in homogeneous chemical reaction by using microwave heating. The specific mode of microwave heating is also used to improve chemical process by the selective and volumetric thermal effect of microwaves on the heterogeneous catalytic reactions.
II. ORIGINAL THERMAL 2.45 GHz MICROWAVE EFFECT
Frequencies ranging from 3 MHz to 30 GHz i.e. from radio-frequencies to the infrared are being used to process food. Depending on the chosen frequency and the particular design of the applicator, treatment by electromagnetic energy at different wavelengths has distinct features. For example, in microwave ovens electromagnetic waves with centimeters wavelength freely propagate and are absorbed by solid or liquid phase food products. The principle of microwave heating is that the changing electrical field interacts with the molecular dipoles and charged ions. The heat generated by the molecular rotation is due to friction of this motion.
The influence of microwave energy on chemical or biochemical reactions is strictly thermal. The microwave energy quantum is given by the usual equation W = h n. Within the frequency domain of microwaves and hyper-frequencies (300 MHz - 300 GHz), the corresponding energies are respectively 1.24 10-6 eV - 1.24 10-3 eV. These energies are much lower than the usual ionisation energies of biological compounds (13.6 eV), of covalent bond energies like OH (5 eV), hydrogen bonds (2 eV), Van der Waals intermolecular interactions (lower than 2 eV) and even lower than the energy associated to Brownian motion at 370C (2.7 10-3eV). From this scientific point of view, direct molecular activation of microwaves should be excluded. Some kind of step by step accumulation of the energy, giving rise to a high-activated state should be totally excluded due to fast relaxation. Like Peterson wrote in many of his articles: "The question and the debate of the non thermal effect of microwave give a lot of damage for the reputation of this technology and its application in industry". Microwaves are only absorbed by dipoles, transforming their energy into heat.
Heat transfer advantages of applying microwave power, a non contact energy source, into the bulk of a material include: faster energy absorption, reduced thermal gradients, selective heating, and virtually unlimited final temperature. For chemical production, the resultant value could include: more effective heating, fast heating of catalysts, reduced equipment size, faster response to process heating control, faster start-up, increased production, and elimination of process steps.
III. MICROWAVE ASSISTED HETEROGENEOUS REACTIONS
Most of the industrial chemical reactions are carried out in a solvent with the presence of solid catalysts. When the reaction is conducted in a heterogeneous medium using a dissipative and/or calatytic solid phase and under microwave heating, the reaction rate increases compared to classical heating under the same conditions (pressure, temperature and reagents concentration). In this paper, three reactions of industrial interest have been studied: Wacker oxidation of cyclohexene with PdCl2 in presence of heptane as solvent, hydrolysis of hexanenitrile to hexanoic acid, and esterification of fatty acids. Granulated ceramics (diameter 5 mm) are used as a catalyst and dissipative solid phase.
When the catalyst is introduced in solid granular form, the yield and the rate of the heterogeneous oxidation, esterification and hydrolysis reactions increase with microwave heating as compared to conventional heating under the same conditions. Table 1 shows an increase yield of 200% for the oxidation and 150% for hydrolysis when the reaction is conducted in the microwave batch reactor (Synthewave 402).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 1: Heterogeneous reactions under microwave and classical heating
The increase in reaction rate corresponds to a virtual difference in reaction temperature. Since the bulk temperature is equal for both the conventional and microwave heated systems, there must be an elevated temperature at the local reaction site: the catalytic surface. This is possible since the heat transfer by microwave depends on the specific loss factor of the different materials: the catalyst and the solvent. Many metallic catalysts are semi-conductors and will readily absorb microwave energy especially at higher temperatures.
The apparent temperature of the catalytic site under microwave irradiation can be estimated from the initial reaction rates. The reaction rate is connected to the temperature by the Arrhenius equation k = A exp (-Eact/RT). For two temperatures, the ratio of the respective reaction rates is related to :
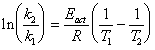 (1)
(1)
From a kinetic analysis of the esterification reaction, the activation energy (Eact) for the hydrolysis of hexanenitrile has been determined to be 70 ± 3 kJ/mole. From the eq. 1, it follows that the apparent elevated temperature of the catalyst for the batch reactions was calculated to be 9 ± 1 K higher than the measured bulk temperature.
In the steady state, the microwave heat transfer to the catalyst is equal to the heat loss of the catalyst to its surroundings. The resulting DT will be linearly dependent on the difference in loss factor and the radius of the catalyst.
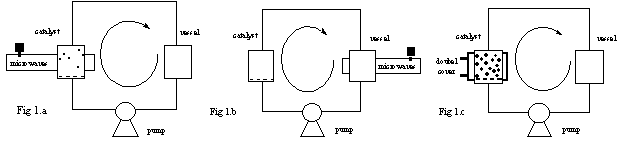
Figure 1 : Microwave and classical continuous reactors
To prove the essential combination of
microwave and catalyst, the continuous flow microwave reactor (figure 1) has
been used in two different set-ups. In the first experimental set-up (Fig. 1a)
the solid catalyst is submitted to microwave irradiation and the liquid is
heated when it passes through the catalyst. In the second experiment (Fig. 1b),
only the liquid is heated by microwaves when it circulates in the cavity vessel
and the catalyst is placed outside the microwave cavity. The temperature was
maintained and checked to be constant throughout the circuit, since the heat
loss to the surrounding occurs mainly in the cavity where it is compensated by
microwave energy. In the third experiment (Fig. 1c), the reaction was conducted
in a conventional continuous reactor operating under the same conditions
(temperature, concentration and pressure) as the microwave continuous
reactor.
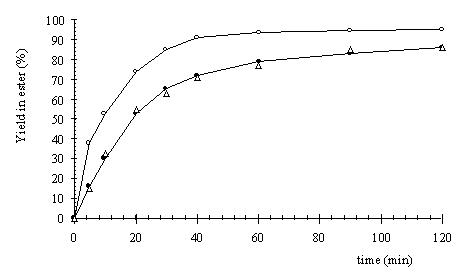
Figure 2: Influence of the location of the catalyst in continuous
microwave reactor
(o ) Fig 1.a
(D ) Fig 1.b (· ) Fig
1.c
In figure 2, the yield is plotted as a function of time for the three experiments at 80 °C and for a flow rate of 1.33 ml/s. Only a direct heating of the catalyst by microwaves (Fig. 1a) gave an increase of initial esterification reaction rate of 150%. Conventional heating of the catalyst vessel (Fig. 1c) and microwave heating with the catalyst outside the cavity (Fig. 1b) gave identical results because the temperature at the catalytic surface was equal to the bulk temperature. Although the bulk temperature in all experiments was the same, there will be a higher temperature at the surface of the catalyst when it is directly heated by microwaves.
IV. MICROWAVE INDUCED SUPERHEATED BOILING OF SOLVENTS
IV.1. Super boiling
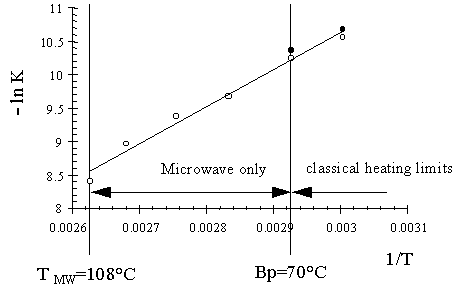
In organic synthesis, it is common practise to carry out reactions under reflux conditions. The boiling ensures a good mixing and the highest possible temperature for the solvent at atmospheric pressure. The temperature of a boiling solvent is normally assumed to be exactly at the point were the partial vapour pressure of the solvent is equal to 1 bar. However, this is not necessarily the case. During reflux, solvent continuously evaporates, condenses and flows back into the reaction pool. Hence, the system is in a steady state rather than in equilibrium and the temperature is not exactly at the equilibrium boiling point. In classical reflux conditions this effect is virtually absent due to the mixing effect of the bubbles that originate from the bottom.
In a microwave heated reactor, the average temperature of the solvent can be at a significantly higher temperature than the atmospheric boiling point. This is due to the fact that the microwave power is dissipated over the whole volume of the solvent, where nucleation points neccesary for boiling are absent. The loss of the excess of thermal energy by boiling can therefore only occur at the side of the reactor or at the solvent-air interface. This results in a reversed temperature profile with a steady average reflux temperature above the classical boiling point. This is called super boiling. With the same reactor overheating is not observed under conventional heating (Mingos and Baghurst, 1992).
The next table shows the super heated temperature for some solvents.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
IV.2. Physical and chemical factors
Homologue series of different kinds of organic solvents were heated by microwaves such as alcohols, acids, amides and nitriles. This study shows that the difference between the microwave superheating and the equilibrium boiling point of the liquid (DTmw) is connected to its chemical and physical properties. The DTmw is non-linearly related to the size of aliphatic moiety of the molecule. This effect is opposite for hydrophilic and hydrophobic solvents.
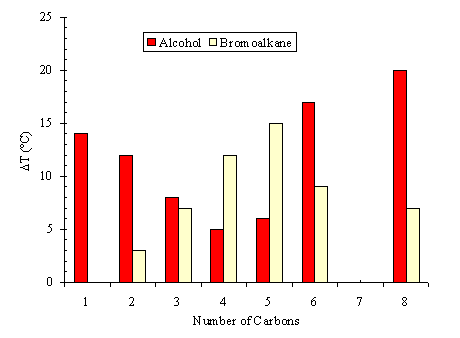
Furthermore we have clearly established that this overheating increases with the free liquid area heated by microwaves. At the microwave overheating point, an avalanche of the temperature is observed with an instantaneous breaking of the free liquid area by surface boiling. Normally, the boiling occurs near the heated surface of conductivity heated reactor wall. However, microwave boiling starts at the free liquid surface. These investigations are confirmed by experiments carried out in multiple microwave devices with different reactor volumes regulated by infrared thermometry.
These conclusions are in contrast with previously published studies in which it was concluded that the microwave nucleation of the vapour bubbles occurs solely at surface pits or scratches of the walls of the container. Under microwave heating the surface of the wall is generally not heated and energy is dissipated inside the bulk liquid. Therefore the temperature at the inner surface of the wall, due to outwards heat transfer, is generally lower than that of the bulk liquid. Boiling only occurs at the free liquid area when the internal energy overcomes the intermolecular attractive forces.
IV.3. CHEMICAL REACTIONS
The microwave superheating phenomena can be exploited to accelerate homogeneous chemical reactions under atmospheric reflux conditions. To allow for kinetic comparisons with conventional heating, we carried out different common industrial reactions (esterification, oxidation...) with careful temperature monitoring by means of infrared thermometry. Temperature probe immersion in the liquid was found to seriously alter the microwave superheating behaviour. Reaction times were reduced from days under classical heating to minutes under microwave heating. The kinetics were analysed as function of the elevated temperature.
This microwave heating mode allows to conduct reactions at
atmospheric pressure with the same yields and reaction times compared to those
conducted in expensive closed reactors (under high pressure) in classical
heating.
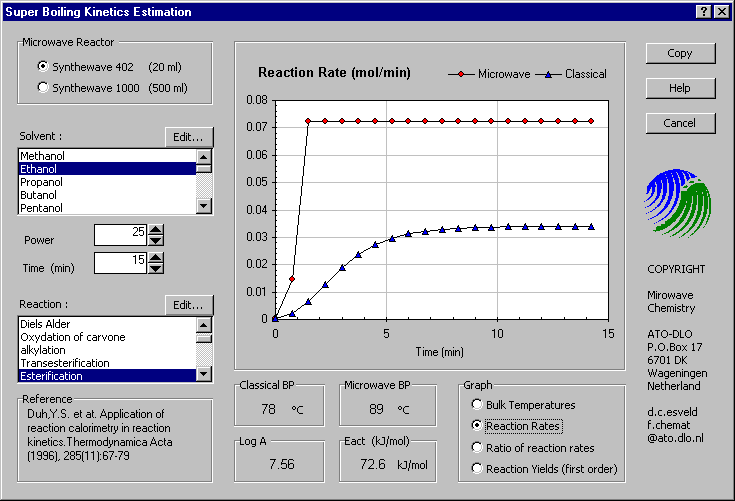
A challenging part of the study was the application of this phenomena at larger scale. The microwave superheating was successfully scaled to a reactor vessel of half a litre. The assumption that the microwave overheating is function of the free liquid area was confirmed. Because of intrinsic fixed microwave wavelength and the criteria for stable operation, it is more profitable to increase the productivity of the reactor by continuous operation instead of upscaling the batch reactor. Therefore we designed a continuous microwave reactor where the superheating effect is employed to accelerate the reaction process. In comparison with the classical process, the volume of the reactor, the resident time, waste reject and the amount of solvent are consequently reduced.
The reaction kinetics for both the microwave and classical heated reactors are based solely on the temperature condition. The maximum temperature in the microwave-heated case is the super heated boiling point. This is dependent on both the solvent and the relative microwave power applied. The estimated value used is based on both experimental data and an heuristic model. The microwave heating rate up to the super heated boiling temperature is strongly dependent on the dielectric properties of the solvent. For different power levels and different reactors, the heating rate is changed according to the power per volume ratio. For the classical reactors a fixed heat conductivity term is set for the different reactor geometries. The surrounding heat basin temperature is equal to the classical boiling point of the solvent. The classical heating rate depends further on the heat capacity of the solvent and the volume of the system.
The temperature and the kinetic parameters of the selected
reaction determine the reaction rate. The rate is calculated according to the
Arrhenius equation and plotted in mol/min. The rate ratio shown is the microwave
over classical reaction rate. For a first order reaction the reaction yield over
time is not dependent on the absolute concentrations. Therefore in this case,
the reaction yields can be calculated from the reaction rates over time. The
time basis can be varied from 5 to 60 minutes.
V. PILOT SCALE EQUIPMENT USED FOR CHEMICAL EXPERIMENTS
Besides a wide variety of household microwave ovens as used by consumers, ATO-DLO has continuous and batch pilot plant microwave ovens for industrial research, both at 2450 MHz (maximum of 6kW). Microwaves can be used in combination with vacuum, overpressure and steam to chemical and physical processes.
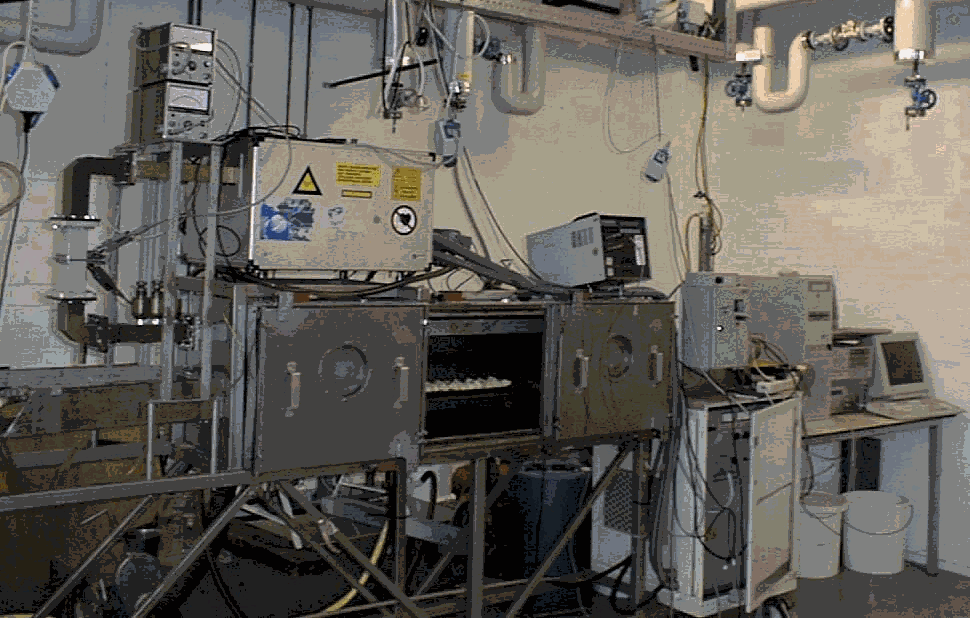
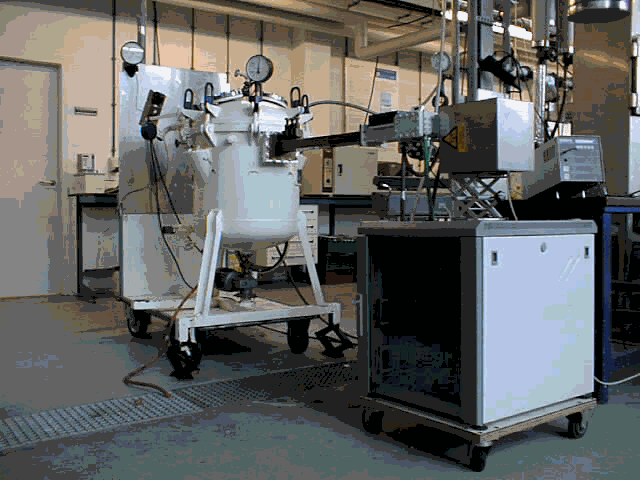
Figure 6: Microwave apparatus at ATO-DLO: left apparatus to use vacuum or increased pressure in combination with microwaves at 2450 MHz; right continuous 2450 MHz for e.g. esterification of fatty acids
VI. CONCLUSION
Microwave overheating is one of the effects, specific for electromagnetic heating, that can be exploited to improve chemical processes. At laboratory scale up to 500ml it has been shown to significantly expand the thermal region normally possible at atmospheric pressure. Although the super heated temperature is strongly dependent on many interrelated factors, an interactive model could be created to assist the scientist in an evaluation of the process parameters.
REFERENCES