[E0019]
Palladium-Catalyzed
Coupling of Silyl-Acetylenes to Aryl Halides Using Microwave
Irradiation
Ulrik
S. S°rensen,
Judith Wede, and Esteban Pombo-Villar*
Nervous
System Research, Novartis Pharma AG, Basel, Switzerland
http://www.mdpi.net/ecsoc-5/e0019/[email protected]
Received:
15 August 2001 / Uploaded 22 August 2001
It
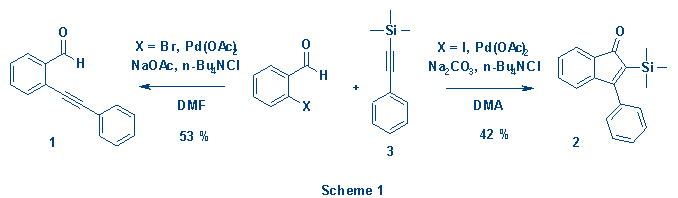
has previously been reported that the reaction between 2-iodobenzaldehyde and
1-phenyl-2-(trimethylsilyl)acetylene (3) in the presence of palladium
acetate gives the corresponding 2-trimethylsilyl-substituted indenone
2.1 When repeating this experiment, using slightly different
reaction conditions and replacing 2-iodobenzaldehyde with 2-bromobenzaldehyde,
we did, however, not isolate the above mentioned indenone but rather the
cross-coupling product 1 (Scheme 1).
This
finding prompted us to study this Sonogashira-type coupling of
1-phenyl-2-(trimethylsilyl)acetylenes to give disubstituted acetylenes. The
Sonogashira coupling is well-known as the synthesis of substituted
acetylenes by coupling aryl or vinyl halides with terminal acetylenes in the
presence of a palladium catalyst and CuI as cocatalyst.2 Numerous
reports describe the coupling of trimethylsilylacetylene with aryl halides in
Sonogashira type reactions, and the trimethylsilyl (TMS) group is generally
unaffected and thus functions as a protecting group which can be subsequently
removed to furnish a new terminal alkyne.3
However,
in the reaction shown above the coupling product 1 is achieved without
previous deprotection of the TMS group of 3. A recent study on the Pd-Cu
cocatalyzed cross-coupling of (arylethynyl)trimethylsilanes and aryl halides or
triflates concluded that copper was required for this reaction to
occur.4 However, the result of our work is a procedure for the
coupling of 1-phenyl-2-(trimethysilyl)acetylenes with arylhalides to give
1-aryl-2-phenyl acetylenes without the use of a copper cocatalyst and
furthermore with short reaction times by the use of microwave5
(MW) irradiation.
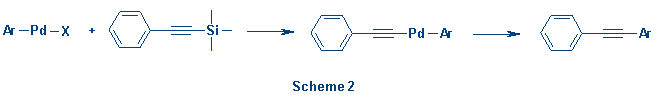
The
mechanism for this transformation is not clear, but one could speculate
that the reaction proceeds via a transmetallation pathway (Scheme 2). A similar
mechanism has recently been argued by Itami et al. to explain the
palladium-catalyzed coupling of alkenyl-silanes with aryl- and vinylhalides in
the presence of tetrabutylammonium fluoride.6 Fluoride-induced
silicon to Pd transmetallation has also been invoked in Pd-catalyzed cross
coupling reactions by Hiyama et al.7
When
the cross-coupling reaction of 3 and 3-iodopyridine was carried out at
room temperature overnight compound 4 was isolated in a moderate 28 %
yield (Table 1, entry 10). To reduce the reaction time and improve the yield we
increased the reaction temperature by means of MW irradiation. The results of
these experiments, aimed at identifying optimized reaction conditions,
are shown in Table 1 and gave compound 4 in up to 90 % isolated
yield.
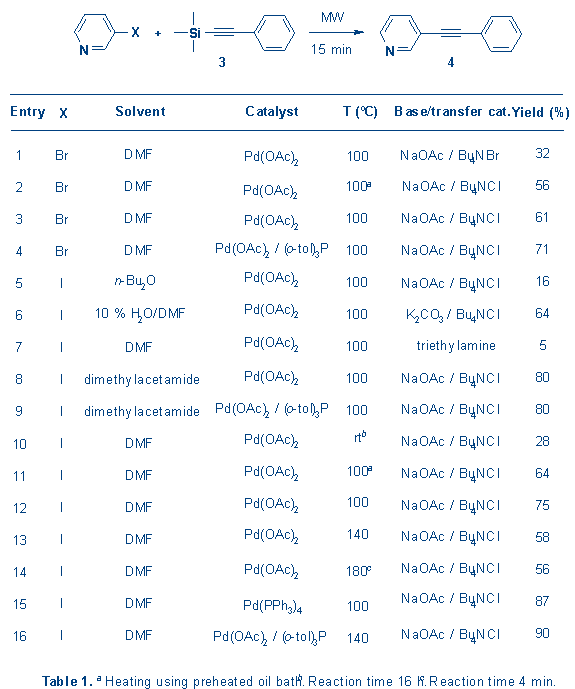
For
most experiments DMF was used as solvent. Being highly polar, it absorbs
microwaves well, resulting in very rapid heating. Using 50 ml of DMF and
irradiation at 450 W we observed a temperature increase from room temperature to
100 ║C within approximately 30 seconds. This rapid heating may explain the
higher yield obtained using MW as compared to conventional heating. Thus,
in an otherwise identical experiment, 4 was isolated in 64 % yield (Table
1, entry 11) when heating on an oil bath as compared to 75 % (Table 1, entry 12)
when applying MW. A similar difference, although less marked, was found when
starting from 3-bromopyridine (Table 1, entry 2 and 3).
Among
the synthesized compounds is the neuroactive compound
2-methyl-6-(phenylethynyl)-pyridine (MPEP, synthesized from 5), a highly
potent and selective metabotropic glutamate receptor
antagonist.8
As
can be seen from both Tables 1 and 2, the addition of
tri(o-tolyl)phosphine in most cases resulted in significantly
improved yields. This could be caused by a stabilizing effect of this ligand on
the reactive palladium species, as previously discussed for e.g. the
palladium-catalyzed Heck coupling.9
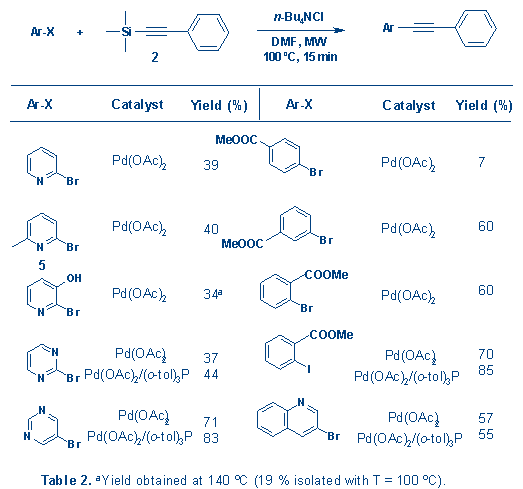
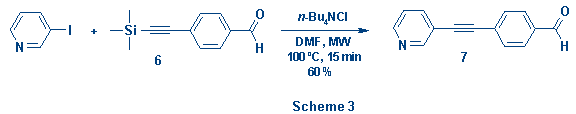
To
broaden the scope of the reaction we are also interested in introducing
functionalized substituents. Thus, in a preliminary experiment, using as
substrate trimethylacetylene 6, it was possible to introduce the
benzaldehyde moiety and obtain the desired coupling product 7 in 60 %
yield (Scheme 3).
In
summary,
we have developed a method for direct coupling of
1-phenyl-2-(trimethylsilyl)-acetylenes with aryl- and heteroarylhalides, to give
disubstituted acetylenes without initial TMS deprotection and without the use of
a copper cocatalyst.
Example
of experimental procedure. Synthesis of 3-(phenylethynyl)pyridine
(4).
3-Iodopyridine (2.50 mmol), 3 (5.00 mmol), Pd(OAc)2 (0.125
mmol), n-Bu4NCl (2.50 mmol), tri(o-tolyl)phosphine
(0.25 mmol), and NaOAc (10.0 mmol) in dry DMF (50 ml) were heated under Ar in
the MW oven10 for 15 minutes at 100 ░C. After cooling, the reaction
mixture was added saturated NaHCO3. Usual work up and silica gel CC
(0-10 % EtOAc in hexane) gave 4 as a dark solid (90 %) which was fully
characterized by 1H-NMR, 13C-NMR, mp, MS (ES+),
and HRMS.
*
Address correspondence to: [email protected] 1) Larock, R. C.;
Doty, M. J.; Cacchi, S. J. Org. Chem. 1993, 58, 4579. 2) a.
Sonogashira, K.; Tohda, Y.; Hagihara, N. Tetrahedron Lett. 1975,
4467. b. (review) Rossi, R.; Carpita, A.; Bellina, F. Org. Prep. Proc.
Int.1995, 27, 127. 3) Takahashi, S.; Kuroyama, Y.;
Sonogashira, K.; Hagihara, N. Synthesis 1980, 627. 4) Nishihara,
Y.; Ikegashira, K.; Hirabayashi, K.; Ando, J.-i.; Mori, A.; Hiyama, T. J.
Org. Chem. 2000, 65, 1780. 5) (recent reviews) a. Caddick, S.
Tetrahedron 1995, 51, 10403. b. Strauss, C. R.; Trainor, R.
W. Aust. J. Chem. 1995, 48, 1665. c. Galema, S. A. Chem.
Soc. Rev.
1997, 26, 233. 6) Itami, K.; Nokami, T.; Yoshida, J.-i. J.
Am. Chem.
Soc.
2001, 123, 5600. 7) Hiyama, T.; Hatanaka, Y. Pure Appl.
Chem. 1994, 66, 1471, and references cited therein. 8) a.
Gasparini, F.; Lingenh÷hl, K.; Stoehr, N.; Flor, P. J.; Heinrich, M.; Vranesic,
I.; Biollaz, M.; Allgeier, H.; Heckendorn, R.; Urwyler, S.; Varney, M. A.;
Johnson, E. C.; Hess, S. D.; Rao, S. P.; Sacaan, A. I.; Santori, E. M.;
Velišelebi, G.; Kuhn, R. Neuropharmacology1999, 38, 1493.
b. Salt, T. E.; Binns, K. E.; Turner, J. P.; Gasparini, F.; Kuhn, R. Br. J.
Pharmacol. 1999, 127, 1057. 9) Beletskaya, I. P.; Cheprakov,
A. V. Chem. Rev. 2000, 100, 3009. 10) All MW experiments
were carried out in a MLS-Ethos 1600 instrument from Milestone except for entry
14 Table 1 which was performed using the PersonalChemistry
SmithCreatorÖ.