[E0020]
Microwave assisted
synthesis of room temperature ionic liquid precursor quaternary
salts
in closed
vessel
Bhushan M. Khadilkar* and
Geeta L. Rebeiro
Applied Organic Chemistry
Laboratory,
University Dept of Chemical Technology, University of Mumbai, Matunga, Mumbai
400 019, India. E-mail: [email protected]
Received: 15 August 2001 / Uploaded 22 August 2001
Various alkylpyridinium and
1-alkyl-3-methylimidazolium halides were synthesized on a large-scale under
microwave irradiation, in a closed vessel. The reaction time was drastically
reduced as compared to conventional methods, and good yields were
obtained.
Key
words: Ionic
liquid, quaternization, microwave, pyridine,
1-methylimidazole.
Room temperature ionic
liquid (RTIL) is no more a new word to a scientific community today. Rising
number of publications are indicative of the potential of RTILs as ‘neoteric
solvents’ for various chemical reactions. These include Friedel-Crafts
reactions1-3, enzyme catalyzed reactions4,5,
hydrogenations6,7, benzoylation8, Heck
reaction9, Fischer indole synthesis10, etc. RTILs are
being looked upon as future commercial11 solvents. The acidic ionic
liquids can act both as catalyst as well as solvent. This dual property of RTIL
has turned out to be a boon in itself to carry out a variety of chemical
transformations and is aptly given the name ‘designer solvent’. RTILs12
known today, are mainly composed of alkylpyridinium or dialkylimidazolium
salt and a Lewis acid.
The preparation of some of
these salts, e.g. 1-butylpyridinium and 1-butyl-3-methylimidazolium chlorides,
required for the widely used RTILs, is quite time consuming. Conventionally it
requires as long as 72 hours of reflux13, 14. Our aim was to reduce
overall time of preparation of the ionic liquids and to synthesize the precursor
salts on a large-scale in shorter time period. While we have been working on
microwave assisted synthesis of these salts, recently we came across a
report15 on the preparation of alkyl-3-methylimidazolium salts under
microwave irradiation. However, there are some major drawbacks of this method.
It is carried out in an open test tube. Hygroscopic nature of salts may not
permit a large-scale preparation by this method. Also the irritant volatile
alkyl halides as well as the corrosive and hygroscopic 1-methylimidazole, are
released inside the microwave cavity and also wasted. Heating volatile materials
in an open vessel in microwave oven can be hazardous.
We report here the simple
and quick method of preparation of alkylpyridinium and
1-alkyl-3-methylimidazolium salts on a large-scale in a closed vessel under
microwave irradiation in CEM make microwave digester, MARS 5.
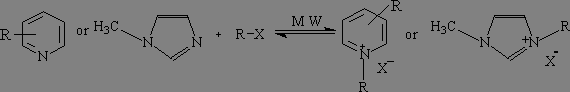
X= Cl,
Br
The quaternization of some
alkyl halides like BuBr and PrBr with 1-methylimidazole took place under reflux
condition in domestic microwave oven. But alkyl halides like BuCl gave only
trace amount of product formation whereas 2-phenylethylchloride, bromohexane,
and 2,6-lutidine did not react under reflux condition under microwave
irradiation at normal pressure. When we carried out the reaction in
closed vessel in a microwave digester, excellent yield of the product was
obtained. The microwave digester used for the reaction has a provision for
recording temperature and pressure during irradiation. In order to investigate
the effect of change in temperature on the reaction, we carried out the reaction
of 1-butyl chloride with pyridine and 1-methylimidazole respectively at three
different temperatures viz. 100 oC, 150 oC and 200
oC. It was observed that in both the cases at 100 oC,
under pressure no product was formed, at 150 oC maximum conversion of
1-butyl-3-methylimidazolium chloride salt was obtained whereas 1-butylpyridinium
chloride was formed only in trace amount. It was only at 200 oC that
the 1-butylpyridinium chloride salt was formed in quantitative yield. The
reaction times were reduced drastically from 72 hours to 1 hour and from 22
hours to 24 min in case of 1-butylpyridinium and 1-butyl-3-methylimidazolium
chlorides respectively. This also shows that 1-methylimidazole reacts faster
compared to pyridine. The thermal profileof thereaction during microwave irradiation is shown
in fig.1and 2. The results are presented in the table below.
The optimized results of
quaternary salts prepared in closed vessel.
|
|
|
|
Time/
min |
oC |
psi |
% | |
|
|
|
|
| ||||
|
|
1-methylimidazole |
1-chlorobutane |
|
|
|
|
|
|
|
1-methylimidazole |
1-bromobutanea |
|
|
|
|
|
|
|
1-methylimidazole |
1-bromopropanea |
|
|
|
|
|
|
|
|
1-chlorobutaned |
+1 |
24 |
200 |
271 |
|
|
|
|
1-bromopropane |
|
|
|
|
|
|
|
|
1-chloropropane |
|
|
|
|
|
|
|
|
1-bromobutaned |
+2 |
3 |
150 |
32 |
|
|
|
|
2-phenethylchloride |
|
|
|
|
|
|
|
|
1-bromohexane |
|
|
|
|
|
|
|
|
1-bromobutane |
|
|
|
|
|
|
|
|
1-chlorobutaned |
+5 |
25 |
230 |
191 |
|
|
|
|
1-bromopropane |
|
|
|
|
|
aReactions were carried out
under reflux in modified domestic microwave oven (Kenstar Model) at 750 W, 30 %
power, irradiated for the time given in table & the MW end temp. noted
down.
bx represents the time set to
reach the given temp. and y, the hold time to continue irradiation at the given
temp.
c represents maximum pressure
reached in the reaction.
dIn case of BuBr & BuCl,
irradiation was done by programming in 2 steps.
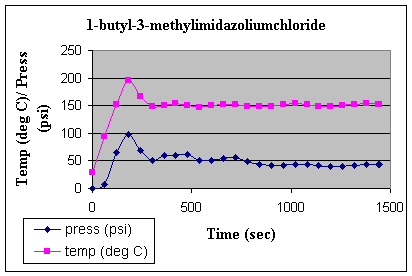
Fig. 1: Thermal profile of
the microwave irradiation of 1-methylimidazole and 1-BuCl.
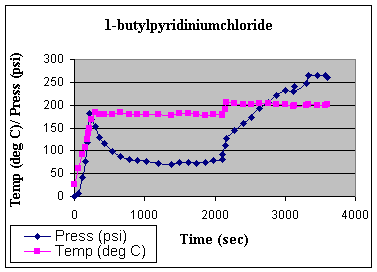
Fig. 2: Thermal profile of
the microwave irradiation of Pyridine and 1-BuCl.
Pyridine (S.d. fine grade)
and 1-methylimidazole (Merck grade) were dried, distilled and stored over KOH
and ethyl acetate was dried over CaH2. The alkyl halides (commercial
grade) were used without further purification.
- Preparation of
1-butyl-3-methylimidazolium chloride (BMIMC):
1H NMR (500 MHz,
DMSO-d6):d = 0.86 (t, 3H, J = 7.37 Hz,
CH3), 1.23 (m, 2 H, CH2), 1.77 (m, 2 H, CH2),
3.9 (s, 3 H, NCH3), 4.22 (t, 2 H, J = 7.1 Hz, NCH2), 7.88
(d, 1 Harom), 7.96 (d, 1 Harom), 9.71 (s, 1
Harom)
13C NMR (125 MHz,
DMSO-d6):d = 13.55, 19.04, 31.78, 35.98, 48.6, 122.64,
123.83, 137.15.
- Preparation of 1-butylpyridinum
chloride (BPC):
1H NMR (300 MHz,
DMSO-d6):d = 0.9 (t, 3 H, J= 7.2 Hz, CH3), 1.3
(m, 2 H, CH2), 1.94 (m, 2 H, CH2), 4.69 (t, 2 H, J = 7.2
Hz), 8.1- 9.23 (m, 5 Harom)
13C NMR (300 MHz,
DMSO-d6):d = 13.22, 18.6, 32.68, 60.1, 127.94, 144.86,
145.39.
We have developed a very
efficient, quick, and practical method for the preparation of alkylpyridinium
and 1-alkyl-3-methylimidazolium salts. The pressure reactor used for the
reaction is very easy to handle and to set up. The time required to synthesize
the salts is reduced by the factor of 72 and 60 in case of BPC and BMIMC
respectively, when compared to conventional method.
The use of a closed vessel
allows for stoichiometric amounts of alkyl halides to be reacted, instead of
excess, with no apparent loss of yield, highlights the ‘green’ aspect of this
improved procedure. It also provides a greener and safer synthesis of the ionic
liquid precursors, the quaternary salts. In the reported method, reaction is
carried out in an open test tube. This poses serious problems of hazards and
also results in wastage of the reactants. Our method overcomes all these
problems and is far safer.
The authors are thankful to
B.R.N.S, Dept of Atomic Energy, Govt. of India; A.I.C.T.E., New Delhi,
India, for financial assistance, and G. D. Gokhale Trust, Mumbai, India
for awarding fellowship to one of the authors.
- Boon JA, Levisky JA, Pflug JL and
Wilkes JS, Friedel- Crafts reactions in ambient- temperature Molten salts.
J. Org. Chem. 51: 480-483 (1986).
- Earle M J, Adams C H, Roberts G
and Seddon KR, Friedel-Crafts reactions in room temperature ionic liquids.
J. Chem. Soc. Chem. Commun. 19: 2097-2098 (1998).
- Song CE, Shim WH, Roh EJ and Chol
JH, Scandium (III) triflate immobilized in ionic liquids: a novel and
recyclable catalytic system for Friedel- Crafts alkylation of aromatic
compounds with alkenes. J. Chem. Soc. Chem. Commun. 17: 1695-1696
(2000).
- Schofer S H, Kaftzik N,
Wasserscheid P and Kragl U, Enzyme catalysis in ionic liquids: lipase
catalyzed kinetic resolution of 1-phenylethanol with improved
enantioselectivity. J. Chem. Soc. Chem. Commun. 5 :425-426
(2001).
- Freemantle M, New Horizons for
ionic liquids. Chemistry and Industry Jan 1, 21-25
(2001).
- Saurez PA, Dullius JE, Einloft S,
De Souza RF and Dupont J, The use of new ionic liquids in two phase catalytic
hydrogenation reaction by Rhodium complexes. Polyhedron 15: 1217-1219
(1996).
- Dyson PJ, Ellis DJ, Parker DG and
Welton T, Arene hydrogenation in a room temperature ionic liquid using a
ruthenium cluster catalyst. J. Chem. Soc. Chem. Commun. 1: 25-26
(1999)
- Khadilkar BM and. Rebeiro GL,
Benzoylation in room temperature ionic liquid.
Synth. Commun : 30, 1605-1608 (2000).
- Hisahiro H, Yumiko S, Takashi H,
Toshio S, Masayoshi A, Keisuke O and Chiaki Y, Heterogeneous Heck reaction
catalyzed by Pd/ C in ionic liquid. Tetrahedron Lett. 42: 4349- 4351
(2001).
- Khadilkar BM and Rebeiro GL,
Chloroaluminate Ionic Iiquid for Fischer Indole Synthesis. Synthesis 3:
370-372 (2001).
- Ionic liquids open up vistas.
Chemistry & Industry, 16th April, pp. 231
(2001).
- Seddon KR, Ionic liquids for Clean
Technology. J.
Chem. Tech. Biotechnol. 68: 351-356 (1997)
- Carpio RA, King LA, Lindstrom RE,
Nardi JC and Hussey CL, Density, Electric Conductivity, and Viscosity of
Several N-Alkylpyridinium Halides and their mixtures with Aluminum Chloride.
J. Electrochem. Soc.: Electrochemical Science and Technology 126:
1644-1649 (1979).
- Wilkes JS, Levisky JA, Robert AW
and Hussey CL, Dialkylimidazolium Chloroaluminate Melts: A New Class of Room
–Temperature Ionic Liquids for Electrochemistry, Spectroscopy, and Synthesis.
Inorg. Chem. 21: 1263-1264 (1982).