[E0021]
HANTZSCH
ESTER SYNTHESIS BY USING AN AQUEOUS HYDROTROPE SOLUTION AS A SOLVENT, IN
CONTINUOUS MICROWAVE REACTOR (CMR)
Bhushan
M. Khadilkar* & Virendra R. Madyar
email: [email protected],
[email protected]
Applied Organic Chemistry
Laboratory, University Department
of Chemical Technology, University of Mumbai, Nathalal Parikh Marg, Matunga,
Mumbai 400 019, India
Received: 15 August 2001 / Uploaded 22 August 2001
After 1995, rapid development in microwave
equipment and modification of ovens was witnessed. To develop microwaves
assisted large scale reaction methodologies has become a need of an hour. We
report here a simple design of continuous microwave reactor (CMR) for carrying
out reactions on a large scale in a domestic microwave oven. We report Hantzsch
ester synthesis, scaled up to 75 gm., using a novel reusable aqueous hydrotrope
solution as a safe alternative to inflamable organic
solvents, in microwave cavity. Separation of the product
was very easy as it separated as a separate solid phase, when cooled outside the
microwave cavity. The solvent was recycled. We could obtain high yields of
dihydropyridines.
Continuous microwave reactor (CMR), scale-up,
aqueous hydrotrope solution, Hantzsch synthesis, dihydropyridines, calcium
channel blocker.
Marked growth in a field of microwave
technology development is evident from number of papers and patents, in the
areas of organic synthesis [1], polymer synthesis [2], material science
[3], food technology [4], environmental technology [5], etc. We have been in the
field and in last five years have published a number of papers.
We have been working [6,7]
in the synthesis of commercially important calcium channel blockers such as
nifedipine, nitrendipine and various other 1,4-dihydropyridines. Some recent
reviews [8-11] are available on dihydropyridine chemistry.
Hydrotropy is the
phenomenon by which otherwise water insoluble compounds can be solubilized in
the aqueous solution of certain compounds like arene sulphonates. These
compounds, known as hydrotopes, are readily water soluble. The aqueous
concentrated solutions (20 to 50 %) of the hydrotopes can significantly increase
water solubility of many water insoluble compounds. The hydrotope solutions can
be safe solvents, as they are non-inflamable, and non-toxic as water is the bulk
medium. Hence the use of aqueous hydrotrope solution can be considered as a
replacement for organic solvents, in a step towards development of
environmentally friendly processes. Though not many reports are available
on hydrotope solutions as reaction media, reactions such as hydrolysis of ester
and oximation of cyclodecanone [12], cross-Cannizzaro reactions of
benzaldehydes and formaldehyde [13], Claisen-Schmidt condensation reactions
[14] have been reported in aqueous hydrotrope solution. Use of inflamable
solvents like methanol, isopropyl alcohol, etc. can be very dangerous for the
use in the microwave cavity.
BATCH PROCESS -
In order to determine the parameters like
resident cavity time of the reaction mixture, suitable hydrotope, etc. for the
continuous reactor synthesis it was necessary to study the reactions in batch
process. We have used for the first time aromatic hydrotrope solution system
such as 50% sodium p-toluene sulphonate aqueous solution (NaPTSA), 40% sodium
cumene sulphonate aqueous solution (NaCuS) and 20% sodium p-xylene sulphonate
(NaXS) aqueous solution to carry out Hantzsch ester synthesis to give high
yields of 4-aryl-1,4-dihydropyridines under microwave
exposure.
We studied two routes (Route A and B) for
Hantzsch ester synthesis to give 4-aryl-1,4-dihydropyridines under microwave
exposure. Initially each route is studied and optimized for a batch process in
50% NaPTSA under microwave exposure. We studied the reaction in 40% NaCuS and
20% NaXS hydrotrope solution too. We found that the yields of DHPs were higher
in 50% NaPTSA than in the other two hydrotrope solutions. The route B was found
more covenient for CMR. Route A requires aq. ammonium hydroxide which is
difficult to handle in CMR.
Dihydropyridine ester synthesis using aqueous hydrotrope solutions under microwave irradiation
Route A

Route B

Optimization for batch
process
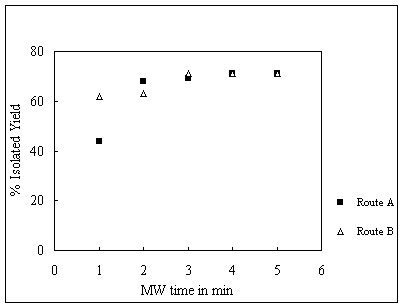
Figure 1 Optimization of DHP synthesis under
microwave exposure in 50% NaPTSA by routes A &
B.
Hantzsch reaction for DHP synthesis was
optimized for batch process in a modified IFB Neutron (760W, 2450MHz) microwave
domestic oven.
 |
|
 |
|
Development of continuous microwave reactor
(CMR) for Scale-up
We have developed two types of reactors to be
used in CMR for our initial study, a glass coil reactor and another a planer,
circular glass reactor (see Figure 3). In case of glass coil reactor resistance
to the flow of reaction solution was observed and also the chances of clogging
of DHP formed during the final stages of reaction was more probable in coil
shaped reactor.
A circular glass reactor was then constructed
for CMR study to avoid the problems observed in the coil shaped reactor. The
reactor was found to be suitable for our study as the reaction solution moved
smoothly and also chances of clogging of DHP formed during the final stages of
reaction was avoided by this kind of reactor design. The glass reactor was
placed in such a way that it traversed along the circumference of the turntable
(glass plate without a rotor) inside the microwave cavity. At the rear wall of
the domestic microwave oven two holes of 1 cm diameter and 5 cm apart from each
other were drilled as inlet and outlet ports for the two ends of glass reactor
to come out. To these ends teflon tubing was connected. The tubes were cooled to
control the temperature during microwave
irradiation.
The reactor volume was 65 ml and that of
connecting teflon tubes was 35 ml (dead volume). Therefore the total volume for
CMR was 100 ml and the reaction was carried out in a closed loop mode. The
reaction mixture was taken in a three-necked round bottom flask of 500 ml
capacity and the mixture was stirred using magnetic needle. A condenser was
fitted to one of the central neck while remaining two were used as inlet and
outlet ports.
Optimization study for MW irradiation time
for nitrendipine synthesis in CMR
The flow rate of 50% NaPTSA with respect to mw
irradiation time was optimized to get controlled heating of the solution. The
best heating profile was obtained at 24 rpm of the pump and flow rate of 100
ml/min can be easily maintained using a peristaltic pump. This homogeneous
solution was then pumped through omega shaped glass reactor via Teflon tubing
using peristaltic pump (Electrolab, model PP-VT 100
series).
The reaction mixture containing equivalent
amounts of 3-nitrobenzaldehyde, ethyl acetoacetate and methyl 3-aminocrotonate
were solubilized in 50 % NaPTSA and circulated through microwave cavity for
known microwave exposure time. It was observed that at 24 min maximum yield for
nitrendipine was obtained (table 3, figure 3).

Figure 3 Schematic diagram for continuous
microwave reactor (CMR)
Table 3 Optimization of MW irradiation time
for nitrendipine synthesis in CMR
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
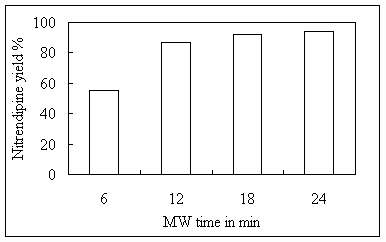
Figure 3 Optimization of MW irradiation time
for nitrendipine synthesis in CMR
 |
|
 |
|
The Hantzsch dihydropyridine ester synthesis
was carried out in aqueous hydrotrope solution using two different
routes.
BATCH
PROCESS
Route
A:
In a round bottom flask an aldehyde (5 mmol)
was added to the hydrotrope solution (7 ml) and solubilized (by warming to
40oC if necessary), to this solution alkyl acetoacetate (10 mmol) and
2 ml ammonia (sp. gravity 0.91) solution were added. The reaction mixture was
then irradiated at full power in a modified domestic microwave oven provided
with reflux and stirring facility. Immediately after the irradiation, reaction
temperature was noted. The reaction was then cooled to room temperature or in
ice as needed, to give 4–aryl-1,4-dihydropyridine ester as the solid product.
The solid product obtained after filtration was washed with 10 ml of water and 5
ml of methanol. The product was air dried which showed the correct melting point
(table 1). Small amount of the product was recovered as a second crop. Yields
reported are combined yields. The completion of reaction was monitored by TLC
(toluene–ethyl acetate 8:2 v/v).
Route
B:
Aldehyde (5 mmol) was added to the hydrotrope
solution (7 ml) and was solubilized by warming when necessary. To this solution
alkyl acetoacetate (5 mmol) and methyl 3-aminocrotonate (5 mmol) were added and
the reaction mixture was irradiated as per the above procedure. The results are
summarized in (table 2).
For all the substrates in the above routes the
amount of hydrotrope was sufficient to solubilize the reactant. Solubility of
reactants (solid aldehydes) in aqueous hydrotrope solution was found by loss in
weight method [12]. The alkyl acetoacetate and methyl 3-aminocrotonate were
completely soluble in the hydrotrope solutions. In both the methods it was noted
that when the same reactions were performed in a water bath under microwave
irradiation (end temperature 860C) they failed to give significant
yields for the comparable time. This supports the utility of microwave
assistance to carry out the reactions.
Table 1 Optimized % yields for the synthesis
of DHPs by route A
|
|
|
|
|
(min.) |
|
NaPTSAa |
|
NaCuSa |
|
NaXSa |
|
Yield |
|
Obsd
[8-11]. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
those of authentic
samples.
Entry no. 1-7 structure
R1= Me; Sr. no. 8 structure
R1
Table 2 Optimized % yields for the synthesis
of DHPs by route B
|
|
|
|
|
|
|
NaPTSAa |
|
NaCuSa |
|
NaXSa |
|
|
|
Obsd [8-11]. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
with those of authentic
samples.
#Entry no. 1-7 structure
R1 = Me; Sr. no. 8 structure R1=
Et
CMR STUDY (for nitrendipine
preparation)
125 ml of 50% NaPTSA solution were introduced
in a 500 ml three necked round bottom flask. To that solution
m–nitrobenzaldehyde (0.15 mole, 22.68 g) was added. The mixture was stirred for
15 min in order to solublize the aldehyde completely. To this clear solution
ethyl acetoacetate (0.15 mole, 19.52 g) and methyl 3–aminocrotonate (0.15 mole,
17.25 g) was added. This homogeneous solution was then pumped through omega
shaped glass reactor via Teflon tubing using peristaltic pump (Electrolab, model
PP-VT 100 series). The flow rate was optimized to 100 ml/min. The reaction
mixture was circulated through microwave cavity in 4 cycles of 6 min each. A 2
min gap between each cycle (only to avoid excessive heating) was imposed. The
reaction mixture was cooled to room temperature and then into crushed ice for 10
min. The solid product obtained after cooling was filtered, washed two times
with 25 ml of water, then by 15 ml of methanol and air dried to give 94 % (50.76
g) yield. The hydrotrope solution can be reused. Similarly, other DHPs were also
synthesized in continuous microwave reactor (CMR) (Table
4).
Table 4. Optimized results for the synthesis
of DHPs by route B in CMR
|
|
|
|
|
|
|
yield
a |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
# Route B: equimolar amount of
aldehyde, alkyl acetoacetate and methyl 3-amino crotonate
in
50% NaPTSA solution. Entry no.
1 structure R1= Et; Sr. no. 2-5 structure R1 =
Me
aYield of pure
isolated product. The melting point and spectral data of products were
identical
with those of authentic
samples.
Nifedipine was obtained in relatively low
yields, as the reaction time was not increased due to development of brown
coloration to the reaction mixture. We have observed that development of such
color effects the quality of the product, moreover o-substituted DHPs are known
to form lower yields.
Following conclusion can be drawn form this
work:
- Aqueous hydrotrope is a green and safe
alternative to organic solvents under MW exposure.
- MW and hydrotrope combination reduces
reaction time, with ease in isolation of products.
- Though the concentration of the aq. hydrotope
solution is high, the products separate out as solid phase and aqueous
hydrotrope solution is reusable.
- Continuous microwave reactor (CMR) helps in
scaling up reaction within a domestic microwave oven, much cheaper than the
commercial systems available.
- Its would be easily possible to
scale up the reaction further by designing an appropriate planer glass
coil.
- The reaction time could be further
reduced by choosing an appropriate planer glass coil.
- Fini A., Breccia A., Chemistry of microwaves;
Pure Appl. Chem., 4 (71), 573-579,
1999.
- Nedelmann H. , Wegel Th., Hicke H. G. ,
Muller J., Paul D., Microwave plasma polymerization of acrylic acid on
poly(ethylene terephthalate) track etched membranes; Surface and Coatings
Tech., (116-117), 973-980, 1999.
- Rao K. J., Vaidyanathan B., Ganguli M.,
Ramakrishnan, Synthesis of inorganic solids using microwaves; Chem.
Mater., 11, 882-885, 1999.
- Ponne C.T., Barlets, Interactrion of
electromagnetic energy with biological material-relation to food processing;
Radiat. Phys. & Chem., 45, 4, 591-607,
1995.
- Van Loock W. M., Overview of microwave and
high frequency energy for hazardous waste processing; MW: Theory and
application in material processing IV, First World Congress on MW Processing,
Edited by Clark D. E., Sulton W.H., Lewis D. A., Ceramic
Transactions, Vol. 80, 619-626, 1997.
- Khadilkar B. M., Gaikar V.G., Chitnavis A.
A., Aqueous hydrotrope solution as a safer medium for microwave enhanced
Hantzsch dihydropyridine ester synthesis; Tetrahedron Lett., 36 (44),
8083-8086, 1995.
- Sadvilkar V. G., Khadilkar B. M., Gaikar V.
G., Aqueous solution of hydrotropes as effective reaction media for the
synthesis of 4-Aryl-1,4-dihydropyridine, J. Chem. Technol. Biotechnol.,
63, 33-36, 1995.
- Natale N. R., Learning from the Hantzsch
synthesis; Chemical Innovation, November, 23-28,
2000.
- Coburn R. A., Wierzba M., Suto M. J., Solo A.
J., Triggle A. M., Triggle D. J., Dihydropyridine antagonists activities at
the calcium channel: A quantitative structure-activity
relationship approach; J. Med. Chem., 31, 2103-2107,
1988.
- Janis R. A., Triggle D. J., New developments
in calcium channel antagonists; J. Med. Chem., 26(6), June, 775-779,
1983.
- Stout D. M., Meyers A. I., Recent advances in
the chemistry of dihydropyridine; Chem. Rev., 82, 223-243,
1982.
- Pandit A., Sharma M. M., Intensification of
heterogeneous reactions through hydrotropy: alkaline hydrolysis of esters and
oximation of cyclododecanone; Chem. Eng. Sci., 42 (11), 2517-2523,
1987.
- Sane, P. V.; Sharma, M. M.,
Cross-Cannizzaro reaction in hydrotrope solution; Synth. Commun., 17
(11), 1331-1339, 1987.
- V. G. Sadvilkar, Samant S. D., Gaikar V. G.,
Claisen-Schmidt reaction in a hydrotopy medium; J. Chem. Technol.
Biotechnol., 62, 405-410, 1995.
Thankful to All India Council for Technical
Education (AICTE), New Delhi, India
for Financial assistance.