DRY MEDIA REACTIONS
M. Kidwai
Department of Chemistry, University of Delhi, Delhi-110007 (INDIA)
Fax :
91-11-7666235 E-mail : mailto:[email protected]
Received: 15 August 2001 / Uploaded 22 August 2001
Introduction
Nowadays, the microwave dielectric heating effect uses the ability of some liquids and solids to transform electromagnetic energy into heat and thereby drive chemical reactions. This in situ mode of energy conversion has many attractions to the chemists [1-2], because its magnitude depends on the properties of the molecules. This allows some control of the materials properties and may lead to reaction selectivity. There is a variety of methods for carrying out microwave assisted organic reactions using domestic or commercial ovens, this is basically known as MORE (Microwave Induced Organic Reaction Enhancement) Chemistry [3]. Microwave heating has not been restricted to organic chemistry as various aspects of inorganic chemistry and polymer chemistry have also been investigated. However, usually the same chemistry (Conventional heating) has been observed when the organic reactions involved were carried out. The difference lies in the choice of reaction conditions : the reaction were carried out in high boiling solvents (Dimethylformamide). Heating is fast, but maximum temperatures were chosen below the boiling point of the solvent in order to avoid solvent evaporation. This one could work in open reaction vessels, could choose small amount of solvent when targeting for solubility at the reaction temperature. Overall Bose [3] claims that the method is more cost effective (only simple glassware needed) and environment friendly (less solvent needed).
To demonstrate the versatility of MORE chemistry, a variety of organic reactions have been done using domestic microwave ovens or commercial ovens.
Solid state reactions :- Generally of three types [4].
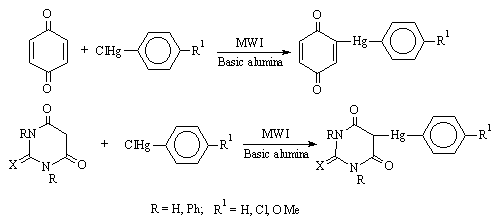
New fungicidal organomercurials of benzoquinone, barbituric acid and thiobarbituric acid with substituted aryl mercuric chloride have been synthesised (Scheme 1) by adsorbing on basic alumina using MWI (Microwave Irradiation) in a few minutes with improved yields [5].

Scheme 1
Reaction of 7-ACA with carboxylic acids by adsorption on basic alumina under MWI for 90-120 sec. (Scheme 2) afforded the 7-substituted cephalosporanic acid derivatives with amidic bond [6].
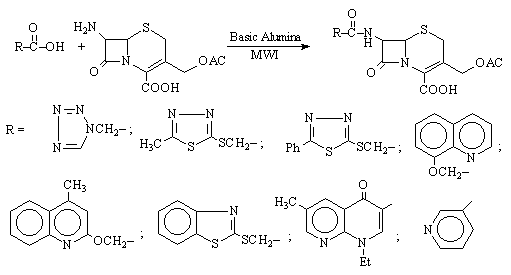
Scheme 2
5-Substituted-2-amino-1,3,4-thiadiazoles were synthesised within 40-80 seconds with improved yield using acidic alumina, they undergo insertion reaction with 5-alkyl-2-mercapto-1,3,4-oxadiazoles to yield the thiadiazolyl substituted triazoles within 40-80 sec. (Scheme 3). A drastic reduction in reaction time and improved yield were observed due to the rapid heating capability of solid support under microwaves [7].
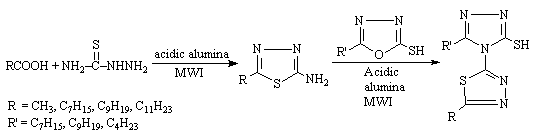
Scheme 3
A series of novel bioactive organotin compounds have been synthesised on basic alumina in an open vessel under MWI (Scheme 4). The reaction time has been brought down from hours to seconds as compared to the conventional heating [8].
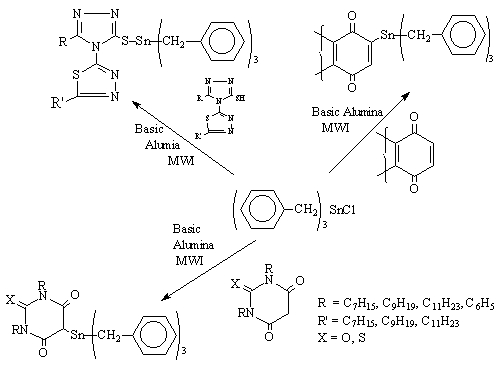
Scheme 4
 Microwave activated
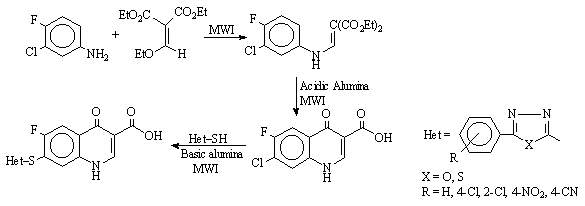
solid support synthesis of new antibacterial quinolones (Scheme 5) was reported
[9].
Microwave activated
solid support synthesis of new antibacterial quinolones (Scheme 5) was reported
[9].
Scheme 5
 A non-conventional
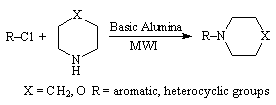
synthetic procedure for nucleophilic aromatic substitution of cyclic amines
using microwaves avoiding catalysts, has been developed (Scheme 6). The obtained
results show the drastic reduction in reaction time and improved yield in solid
phase reaction compared to other methods.
A non-conventional
synthetic procedure for nucleophilic aromatic substitution of cyclic amines
using microwaves avoiding catalysts, has been developed (Scheme 6). The obtained
results show the drastic reduction in reaction time and improved yield in solid
phase reaction compared to other methods.
Scheme 6
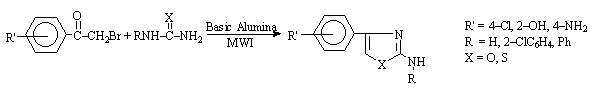 2-Aminothiazoles and
oxazoles have been synthesised (Scheme 7) using solvent-free microwave
technique, the reaction time has been drastically reduced with improved yield as
compared to conventional method [11].
2-Aminothiazoles and
oxazoles have been synthesised (Scheme 7) using solvent-free microwave
technique, the reaction time has been drastically reduced with improved yield as
compared to conventional method [11].
Scheme 7
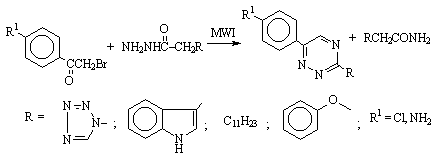 An environmentally
benign, solventless synthesis of 1,2,4-triazines using microwaves is reported
(Scheme 8). Results showed that neutral alumina is the best support in terms of
yield and time for the synthesis of 1,2,4-triazines [12].
An environmentally
benign, solventless synthesis of 1,2,4-triazines using microwaves is reported
(Scheme 8). Results showed that neutral alumina is the best support in terms of
yield and time for the synthesis of 1,2,4-triazines [12].
Scheme 8
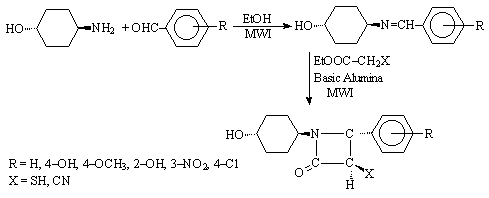 N-(4-hydroxycyclohexyl)-3-mercapto/cyano-4-arylazetidine-2-one were synthesised
from N-(4-hydroxycyclohexyl)-arylaldimine by reacting with ethyl a-mercapto/a-cyanoacetate on basic
alumina under microwaves (Scheme 9), with reduction in reaction time and
improved yield [13].
N-(4-hydroxycyclohexyl)-3-mercapto/cyano-4-arylazetidine-2-one were synthesised
from N-(4-hydroxycyclohexyl)-arylaldimine by reacting with ethyl a-mercapto/a-cyanoacetate on basic
alumina under microwaves (Scheme 9), with reduction in reaction time and
improved yield [13].
Scheme 10
References