Microwave Catalyzed Reactions of H-Dimethylphosphonate with Oxiranes
S. Munavalli1, D. K. Rohrbaugh2, F. J. Berg2, F. R. Longo1 and H. D. Durst2
1Geo-Centers, Inc., P. O. Box 68, Gunpowder Branch, APG, MD 21010 (USA) and
2US Army Edgewood Chemical Biological Center, Aberdeen Proving Ground, MD 21010
(USA)
Phone: 410-436-2819; Fax: 410-436-3764; [email protected]
Received: 15 August 2001 / Uploaded 22 August 2001
Abstract:
Microwave catalyzed reactions of H-dimethylphosphonate with 1, 2-epoxydecane, 5, 6-epoxy-1-hexene, 1, 2-epoxybutane and cyclohexene oxide have been found to cause oxirane ring opening, deoxygenation and hydrophosphorylation. 1, 2-Epoxydecane gave 3 pairs of isomeric products and another arising from the loss of a two-carbon fragment, while 5, 6-epoxy-1-hexene, cyclohexene epoxide and 1, 2-epoxybutane yielded 10, 7 and 3 compounds, respectively.
Keywords: H-dimethylphosphonate, oxirane ring cleavage, deoxygenation, hydrophospho-rylation
Introduction:
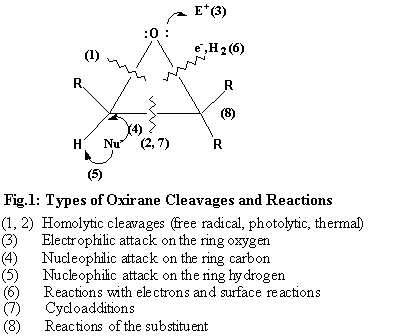
Oxiranes comprise an extremely versatile group of intermediates and as such have attracted considerable attention.1 Because of their ready availability and exceptional reactivity, the epoxides have found varied applications in synthetic organic chemistry. The oxirane ring can be opened under almost all conditions: electrophilic, nucleophilic, neutral, gas-phase, thermal and free radical conditions (Fig.1).1a An excellent review on the preparation and synthetic applications of the oxiranes has appeared.1f Recently we investigated the free radical cleavage of styrene oxide with trifluoromethylthiocopper and reported the formation of products arising from the C – C and C – O bond fission.2 However, their reaction with phosphorus compounds has found only a limited application including their routine use in the Michaelis-Becker reaction.3, 4 Tri-coordinated pentavalent phosphorus compounds or in situ generated intermediates are known to react with oxiranes.3, 4 Thus, phosphorus azide reacted with propylene oxide to give cyclic oxazaphoranes as well as acyclic compounds.5 Also, highly reactive metaphosphate intermediates have been described to open the oxirane ring to yield isomeric 1, 3, 2-dioxaphospholane-2-oxide derivatives.6
The in situ generated electrical energy from microwaves has been used to thermally catalyze chemical reactions. This type of energy transfer depends on the nature and properties of the reacting molecules.7 Since the advent of commercially available microwave cookers, the microwave thermal process is finding increasing and interesting applications in synthetic organic chemistry.8 The popularity of the microwave-induced chemistry appears to rest primarily on its dramatic reduction of the reaction time and the possibility of carrying out reactions in solid phase. In fact, the latter appears to have significantly contributed to its enhanced use. In continuation of our interest in the chemistry of the oxirane cleavage reactions2, 10, the microwave catalyzed oxirane ring opening in the presence of H-dimethylphosphonate has been examined and observed to lead to unusual products. This paper presents the probable mechanism of the formation of various compounds and their GC-MS characterization.

Results and Discussion
Recently, H-phosphonates have attracted considerable attention and have found useful applications in phosphorylation reactions.11 The presence of the readily removable hydrogen at the phosphorus center appears to be the genesis of its reactivity.12 Among other things, H-phosphonates are known to be involved in the addition to: (i) multiple bonds13a and (ii) carbonyl group13b and (iii) in trans-esterifications.13c Dealkylation reactions have also been recorded.13b Both P – O and C – O bond cleavages have been observed.13d Methyl radicals have been stated to react with trimethylphosphite, albeit sluggishly, to give trimethylphosphonate.14 What is unusual about the reaction described here is the non-specific radical formation from H-dimethylphosphonate. ‘Lesser regioselectivity’ observed in the formation of various products has been attributed to ‘the greater length of the carbon-phosphorus bond as compared to the carbon-carbon bond’.
Deoxygenation of organic peroxides with phosphites has been described.15a - c Phosphoranes exhibit reducing properties.15d – e However, epoxides are said to remain unaffected in the presence of phosphites.16 It has also been stated that phosphites17a and phosphines17b deoxygenate epoxides to furnish alkenes. There seems to be some contradiction and uncertainty as regards the reaction of epoxides with phosphorus compounds. Phosphorus stabilized carbanions yield various products on treatment with oxiranes. Thus, the formation of alkenes, cyclopropanes and ketones has been rationalized.18a Significant formation of ketones was observed via hydrogen migration.18b However, the reaction of styrene oxide with benzylidene trimethylphosphorane yielded (2-phenylethyl)ketone as the minor product and cis/trans 1,3-diphenylpropene as the major product.18b With methylenetriphenylphosphorane, styrene oxide furnished a ketone and triphenylphosphine. With strongly basic ylides, cyclic ethers were obtained.18d The reaction of styrene oxide with ethoxycarbonyl triphenylphosphorane gave cyclopropanoids.18e-f Cyclohexene oxide itself reacts with ylide to yield a mixture of olefins via ring contraction.18f
The reaction of oxiranes with phosphines generally leads to the creation of a carbon-carbon double bond between the two carbon atoms involved in the oxirane ring.19 The oxirane ring is cleaved by phosphines in acidic media (cf. Ref. 5, p.119). When diphenylphosphinous azide was caused to react with propenylene oxide, four compounds - two acyclic and two cyclic oxazophospholanes - were formed.4 In the above-cited compounds, the phenyl ring was found to have migrated from phosphorus to nitrogen. The loss of C2H5-moiety from C2H5O-group attached to phosphorus was noticed during the synthesis of oxaphospholanes.7 Ring contraction was observed when cyclohexene epoxide was treated with ethoxy carbonylphosphorane20, while the same Wittig reagent transformed the oxirane ring into a cyclopropyl ring.20 Epoxides yield oxaphospholane with metaphosphates and phosphates.5, 21 Epoxides are formed along with trialkylphosphates when dihydro-1,3-2-dioxaphospholanes and dihydro-1,4,2-dioxophospholanes are heated.22 Free radical reactions of phosphorus compounds have been described.19a, 23
 Microwave induced reaction of H-dimethylphosphonate
(1) with 1, 2- epoxydecane (2) and cyclohexene oxide (3)
has been found to yield products arising from ring cleavage, deoxygenation and
addition. Thus, 1,2-epoxydecane (2) gave 3 pairs of isomeric products
(4 ~ 9, Fig.2) and another compound arising from the loss of a two-carbon
fragment (10). While cyclohexene oxide (3) furnished seven
compounds (11 ~ 16, Fig.2). H-dimethylphosphonate (1)
itself gives two compounds, namely trimethylphosphonate and trimethylphosphate.
There is nothing unusual about these compounds, for they are usually formed
during the oxidation and/or free radical reactions of H-dimethylphosphonate
(1). The mass spectral breakdown of dialkylphosphonates has been
described in detail.24
Microwave induced reaction of H-dimethylphosphonate
(1) with 1, 2- epoxydecane (2) and cyclohexene oxide (3)
has been found to yield products arising from ring cleavage, deoxygenation and
addition. Thus, 1,2-epoxydecane (2) gave 3 pairs of isomeric products
(4 ~ 9, Fig.2) and another compound arising from the loss of a two-carbon
fragment (10). While cyclohexene oxide (3) furnished seven
compounds (11 ~ 16, Fig.2). H-dimethylphosphonate (1)
itself gives two compounds, namely trimethylphosphonate and trimethylphosphate.
There is nothing unusual about these compounds, for they are usually formed
during the oxidation and/or free radical reactions of H-dimethylphosphonate
(1). The mass spectral breakdown of dialkylphosphonates has been
described in detail.24
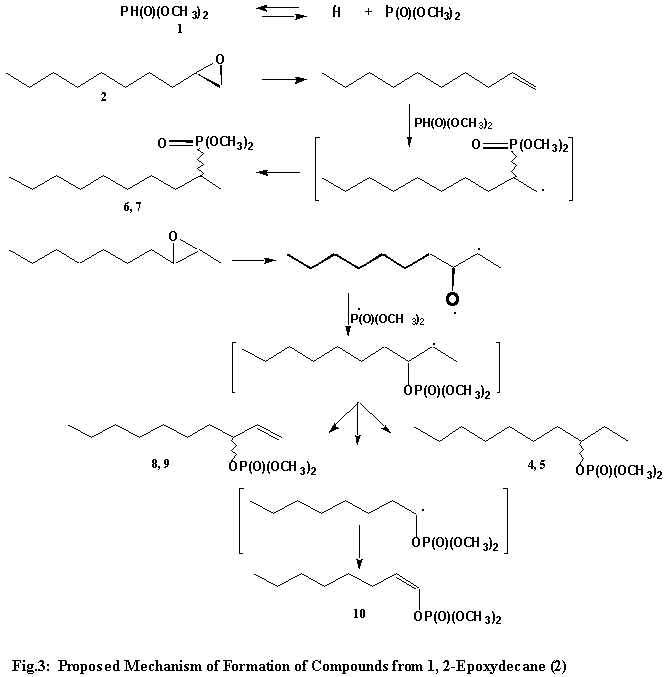
The formation of the isomeric pair of O-(3-decyl)-O,O-dimethylphosphates (4 and 5) can be ascribed to the presence of 2,3-epoxydecane in the starting material. This inference was supported by the GC-MS analysis of the starting material, which indicated the presence of isomers having the same molecular weight as 1, 2-epoxydecane (2) but different retention times on the g. c. column. O-(3-?ecyl)-O,O-dimethylphosphates (4 and 5, Fig. 3) simply arise from the opening of the oxirane ring to form the oxy radical intermediate, which reacts with the phosphonyl radical, followed by hydrogen abstraction. Compounds 6 and 7 are isomers of O-(3-decyl)-O,O-dimethylphosphates (4 and 5) and can be considered to have been formed from
1, 2-epoxydecane (2) via a similar process.
The third pair of isomers, namely O-3-(1-decenyl)dimethylphosphates (8 and 9) are formed from the same free radical intermediate that is involved in the formation of O-(3-decyl)-O,O-dimethylphosphates (4 and 5). In this case, instead of hydrogen abstraction, hydrogen loss occurs to furnish the compounds in question. Finally, the formation of dimethyl (1-octenyl)phosphonate (10), can be rationalized as having been formed from the above mentioned free radical intermediate, which splits off a two carbon entity to give an octyl radical. The octyl radical in turn loses hydrogen to give compound 10. Such vinylphosphonates are known.25 The
various processes described above are shown in Fig.3.
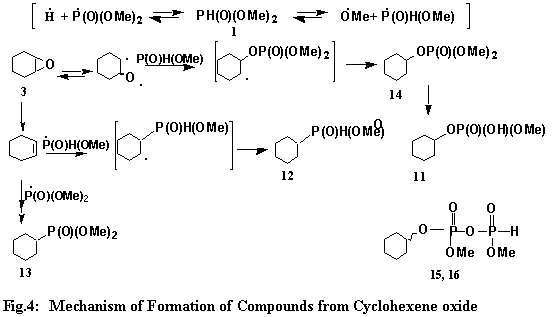
The reaction of H-dimethylphosphonate (1) with cyclohexene oxide (3), contrary to the latter’s
reaction with stabilized phosphorane carbanions, proceeds via free radical processes to furnish products 11 ~ 16 (Fig. 2). The phosphonyl radical adds to cyclohexene, undoubtedly formed from 3 via the deoxygenation, to give a radical intermediate which then simply abstracts hydrogen to yield 13. The phosphonyl radical reacts with the oxy radical resulting from the oxirane ring opening to give radical intermediate, which after hydrogen abstraction yields O-cyclohexylphosphate (14, Fig.4). Then 14 loses a methylene moiety to form O-cyclohexyl-O-methylphosphate (11). Or loses a methyl moiety to form an ‘oxy radical’, which subsequently abstracts hydrogen to form the compound in question. There are precedents for such a loss of an alkyl group from an alkoxy group attached to phosphorus.7 Compounds 15 and 16 are pyrophosphate derivatives. It would be safe to assume that compound 11 serves as the precursor for 15 and 16. Cyclohexeyl methylphosphinate (12) results from the reaction of the in situ formed cyclohexene and methylphosphinyl radical. The former happens to be a product of deoxygenation, while the latter seems to have its origin in H-dimethylphosphonate (1). The recent characterization of a methoxylated product from the reaction of alkyl epoxide with H-dimethylphosphonate (1) lends support to this contention.26 This non-regioselective formation of these compounds may be attributed to the "greater length of the carbon-phosphorus bond as compared to the carbon-carbon bond".24b
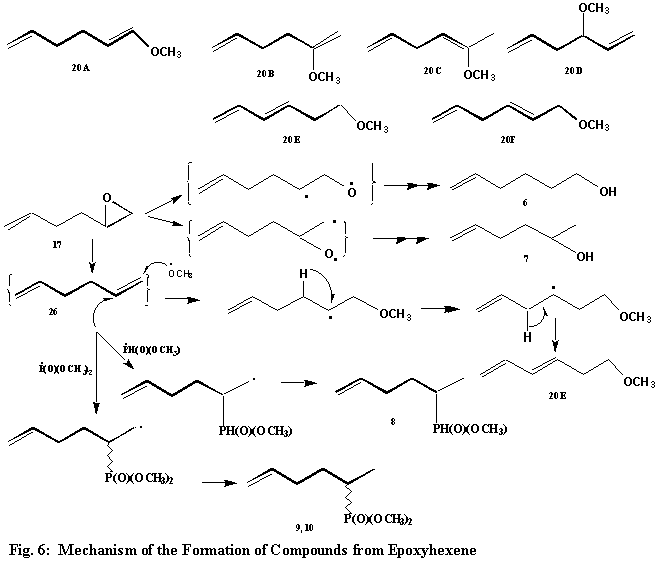
The reaction of H-dimethylphosphonate (1) with 5, 6-epoxy-1-hexene (17) gives 8 compounds (20 ~ 25, Fig.5), excluding the two formed from the former. For compound 20, six structures (20A-20F, Fig.6) were considered. Based on its mass spectral fragmentation pattern, structure 20E was assigned to this compound. Of the six structures, 20E alone can give rise to ions m/e = 59 and 45 corresponding to fragments -CH2CH2OCH3 and -CH2OCH3 respectively. Its formation can be rationalized on the basis of the attack by the methoxy radical on the terminal carbon atom of the in situ formed 1, 5-hexadiene (26, Fig.6) accompanied by the C3-hydrogen migration and followed by the loss of hydrogen to furnish the conjugated dienic derivative, 20E, Fig.6). Two hexenols (21 and 22) were characterized by their mass spectral breakdown patterns. The mass spectrum of compound 21 shows the presence of two ions, m/e = 45 and 59, resulting from a- and b-fragmentation. Compound 22 readily loses water and does not give the above mentioned ions. The oxirane ring of 5. 6-epoxy-1-hexene (17) (Fig. 5) can undergo fission to form the oxy-radical intermediates, which abstract hydrogen to give the hexenols (21 and 22).
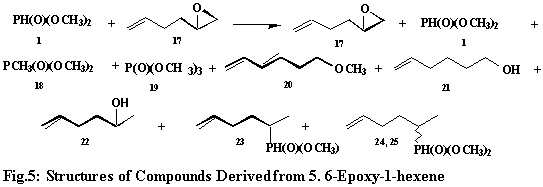
Hydrogen 2-(5-hexenyl) methylphosphinate (23) results from the attack of the phosphinyl radical on the C2-of 1,5-hexadiene (26), which is formed from deoxygenation of 5, 6-epoxo-1-hexene (17). The last two compounds, namely dimethyl 2-(5-hexenyl)phosphonates (24 and 25) represent a pair of stereomers resulting from the attack of the phosponyl radical on the C2-of 1,5-hexadiene (26), followed by hydrogen abstraction by the resultant radical intermediate (Fig. 6). Both trimethylphosphonate and trimethylphosphate (18 and 19) are routinely formed from H-dimethylphosphonate (1) during the oxidation reactions. Their mass spectral breakdown has been discussed.25 The formation and properties of various phosphorus radicals, namely phosphinyl, phosphony and phosphoranyl, have been described.23i –j, 24
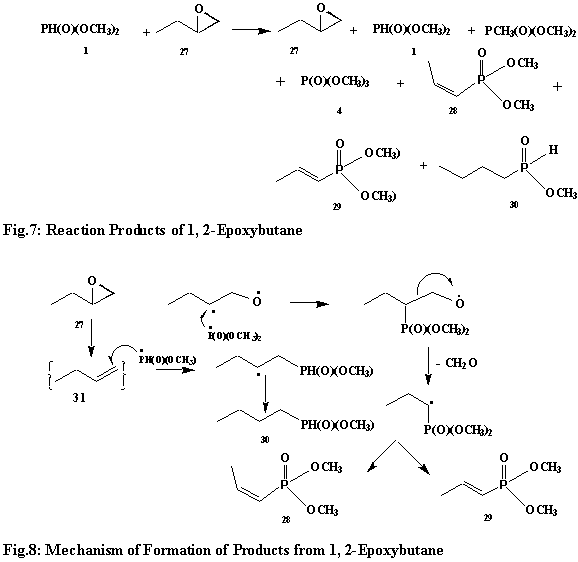
The reaction of H-dimethylphosphonate (1) with 1, 2-epoxybutane (27, Fig.7) appears to be rather simple and straightforward. It gives only 3 compounds: (a) cis- and trans-dimethyl 1-(1-propenyl)phosphonates (28 and 29) and (b) n-butyl dimethylphosphonate (30). The formation of 28 and 29 can be ascribed to the fragmentation of formaldehyde from the radical intermediate formed by the reaction of the phosphonyl radical with the radical intermediate arising from epoxide ring opening. This radical intermediate then goes on to lose hydrogen to give compounds 28 and 29. The mass spectra of 28 and 29 are very similar and have an abundant number of odd mass ions. The latter is said to be a characteristic of monoalkenes.27 The formation of 30 is straightforward. It arises via the addition of the phosphonyl radical to the in situ formed 1-butene (31), followed by hydrogen abstraction. Fig.8 describes the above observations and T3 summarizes the mass spectral fragmentation.
?
Experimental:
Mass spectra were obtained using a Finnigan TSQ-7000 GC/MS/MS equipped with a 30 m x 0.25 mm. i.d. DB-5 capillary column (J and W Scientific, Folsom, CA). The conditions on the TSQ-7000 were: oven temperature 60-270° C at 15° C/min, injection temperature 220°, interface temperature 250° C, source temperature 150°, electron energy 70 eV (EI) or 200 eV (CI) and emission current 400 mA (EI) or 300 mA (CI) and scan time 0.7 sec. Data was obtained in both the electron ionization mode (range 45-450 da) and chemical ionization mode (mass range 60-450 da). Ultrahigh purity methane was used as the CI agent gas with a source pressure of 4 Torr. Routine GC analyses were accomplished with a Hewlett-Packard 5890A gas chromatograph equipped with a J and W Scientific 30 m x 0.53 mm i.d. DB-5 column (J and W Scientific, Folsom, CA). The NMR spectra (1H and 13C) were recorded in CDCl3 with TMS as the internal standard on a Varian VXR-400S spectrometer at 100 MHz and 376 MHz respectively.

Stoichiometric amounts of the respective reagents were mixed in glass vials, vigorously shaken on a vibro-mixer and heated in the microwave oven for a specified period. The reaction mixture was allowed to come to ambient temperature, the cooled product was first analyzed by gas chromatography and then subjected to GC-MS analysis.
Reaction of Hydrogen dimethylphosphonate (1) with 1, 2-Epoxydecane (2, Fig.2): Stoichiometric amounts of hydrogen dimethylphosphonate (1, 0.22 g, 2 mmol) and 1, 2- epoxydecane (2, 0.312 g, 2 mmol) were mixed in a glass vial, stirred for a few minutes using the vibro-mixer and then heated in a table top microwave oven at power 8 for two minutes. The reaction mixture after cooling was analyzed by gas chromatography. Then, it was heated again for four minutes and again analyzed by gas chromatography. When no additional peaks showed up in the chromatogram, it was then subjected to GC-MS analysis. Thus, a total of seven compounds; O-(3-decyl)-O,O-dimethylphosphates (4, 5), dimethyl (1-octenyl)phosphonate (10), 2-(decyl)-O,O-dimethylphosphonate (6, 7), O-3-(1-decenyl)di-methylphosphates (8, 9) excluding compounds containing only the phosphorus moieties, were characterized based on their mass spectral fragmentation behavior. The mass spectral data is given in T1.
Reaction of H-dimethylphosphonate (1) with Cyclohexene oxide (3, Fig. 2): Stoichimetric amounts of H-dimethylphosphonate (1, 0.22 g, 2 mmol) and cyclohexene oxide (3, 0.196 g, 2 mol) were mixed in a glass vial, stirred for a few minutes using the vibro-mixer and then heated in a table top microwave oven at power 8 for four minutes. The reaction mixture after cooling was analyzed by gas chromatography. Then, it was heated again for four minutes and re-analyzed by gas chromatography. When no additional peaks appeared in the chromatogram, the reaction product was subjected to GC-MS analysis. Thus, a total of six compounds: O-cyclohexyl-O-methyl-phosphate (11), cyclohexyl O-methylphosphinate (12), O-cyclohexyl)-O,O-dimethylphosphate (13, 14) and O-[(O-cyclohexyl)-O-methyl]-phosporyl-O-methyl-phosphonate (15, 16) excluding hydrogen dimethylphosphonate, trimethylphosphonate and trimethylphosphate; were characterized based on their mass spectral fragmentation behavior. The structure of one compound remains undetermined as it undergoes extensive breakdown in the mass spectrometer giving no molecular ion peak. The mass spectral data is given in T1.
Reaction of H-dimethylphosphonate (1)
with 5, 6-Epoxy-1-hexene (17, Fig.5): Stoichiometric amounts
of 5, 6-epoxy-1-hexene (17, 0.19 g, 2 mmol) and
H-dimethyl-phosphonate (1, 0.22 g, 2 mmol) were mixed in a glass vial,
stirred for a few minutes using the vibro-mixer and then heated in a table top
microwave oven at power 7 for 90 seconds; 30 seconds each time to a total of 3
times. The reaction mixture after cooling was analyzed by gas chromatography.
Then, it was heated again for four minutes and reanalyzed. When no additional
peaks showed up in the chromatogram, it was then subjected to GC-MS analysis.
Based on their mass spectral fragmentation behavior, a total of eight compounds
excluding the starting materials have been characracterized: (1)
trimethylphosphonate (18), (2) trimtheyl-phosphate (19), (3)
1-methoxy-3, 5-hexadiene (20), (4) 5-hexene-1-ol (21), (5)
5-hexene-2-ol (22), 2-(5-hexenyl) methylphosphinate (23) and (7)
2-(5-hexenyl) dimethylphosphinates (24 - 25). The mass spectral
data is given in T2.

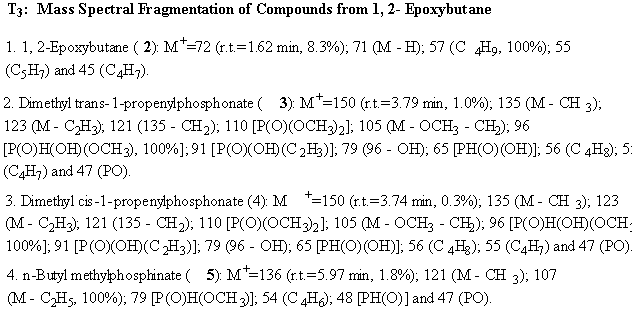
Reaction of H-dimethylphosphonate (1) with 1, 2-Epoxybutane (27, Fig.7): Stoichiometric amounts of 1, 2- epoxydecane (2, 0.14 g, 2 mmol) and H-dimethylphosphonate (1, 0.22 g, 2 mmol) were mixed in a glass vial, stirred for a few minutes using the vibro-mixer and then heated in a table top microwave oven at power 7 for 2 minutes; 30 seconds each time to a total of 4 times. The reaction mixture after cooling was analyzed by gas chromatography. Then, it was heated again for four minutes and reanalyzed. When no additional peaks appeared on the chromatogram, it was then subjected to GC-MS analysis. Based on their mass spectral fragmentation behavior, a total of seven compounds: including the starting materials and two derived from H-dimethylphosphonate were characracterized: (1) trimethylphosphonate (18), (2) trimtheylphosphate (19), (3) dimethly cis-1-propenylphosphonate (28), (4) dimethly trans-1-propenylphosphonate (29), (5) n-butyl methylphosphinate (30). The mass spectral fragmentation data is given in T3.
References:
1. (a) L. G. Lewis in "Comprehensive Heterocyclic Chemistry", vol.7. , A. R. Katritzky, C. W. Rees (series eds), W. Lwawoski (ed.), Pergamon Press, New York, 1984, p.100; (b) J. G. Buchanon, H. Z. Sable in "Selective Organic Transformations", vol. 2, B. S. Thyagarajan (ed), Wiley, New York, (1972), p. 1; (c) M. Bartok and K. C. Long in "The Chemistry of Ethers, Crown Ethers, Hydroxy Groups and their Sulfur Analogs", Part 1, Suppl., S. Patai (ed), Wiley, New York, (1980), p.609; (d) G. Smith, Synthesis 629 (1984); (e) C. Bonini, R. DiFabio, G. Sotgiu, and S. Cavgnero, Tetrahedron 45, 2895 (1989); (f) A. S. Rao, S. K. Paknikar and J. G. Kirtane, Tetrahedron 39, 2323 (1983); (g) K. Maruko, M. Hasegawa, H. Yamamoto, K. Suzuki and G. Tsuchihashi, J. Am. Chem. Soc. 108, 3827 (1986); (h) K. Maruko, S. Nagahara, T. Ooi, and H. Yamamoto, Tetrahedron Lett. 30, 5607, 1989; (i) C. Bonini and G. Righi, Synthesis, 225 (1994).
2. S. Munavalli, D. K. Rohrbaugh, D. I. Rossman, L. R. McMahon and H. D. Durst, J. Organometal. Chem. 587, 160 (1999).
3. A. G. Rowley in "Organophosphorus Reagetns in Organic Synthesis’, J. I. G. Cadogan, Academic Press, NewYork (1979), p.306.
4. A Guide to Organophsophorus Chemistry, L. D. Quin, Wiley-Interscience, New York (2000).
5. G. Bertrand, J.-P. Majoral and A. Baceiredo, Tetrahedron Lett. 21, 5015 (1980).
6. R. Bodalski and L. D. Quin, J. Org. Chem. , 56, 2666 (1991).
7. D. M. P. Mingos and D. R. Baghurst, J. Chem. Soc., Chem. Soc. Rev. 20, 1 (1991).
8. (a) R. J. Gedye, F. Smith, K. Westaway, H. Ali, L. Baldisera, L. Laberge and J. Rousell, Tetrahedron Lett. 27, 279 (1986); (b) R. J. Giguere, T. L. Bray, S. M. Duncan and G. Majetich, Tetrahedron Lett. 27, 4945 (1986); (c) A. Abramovitch, Org. Prep. Proc. Int. 23, 685 (1991); (d) S. Caddick, Tetrahedron 51, 10403 (1995); (e) P. de la Cruz, E. Diez-Barra, A. Loupy and F. Langa, Tetrahedron Lett. 37, 1113 (1996); (f) A. Dandia, H. Teneja, R. Gupta and S. Paul, Synth. Comm. 29, 2323, 1999; (g) B. K. Banik, M. S. Manhas, S. N. Newaz and A. K. Bose, Bioorg. Med. Chem. Lett. 31, 2363 (1993).
9. (a) P. Kumar and K. C. Gupta, Chem. Lett. 635 (1996); (b) S. Jolivet, S. A.-E. Ayoubi, D. Mathe, F. T. Boullet and J. Hamelin, J. Chem. Res(S), 300 (1996).
10. (a) S. Munavalli, D. I. Rossman, D. K. Rohrbaugh and H. D. Durst, National Meeting, American Chemical Society, Anheim (CA) (1995); (b) S. Munavalli, D. I. Rossman, D. K. Rohrbaugh and H. D. Durst (under preparation); (c) S. Munavalli, D. K. Rohrbaugh, F. R. Longo, F. J. Berg and H. D. Durst, 213th National Meeting, American Chemical Society, San Diego (CA) (2001); (d) D. K. Rohrbaugh, S. Munavalli, G. W. Wagner and H. D. Durst (in press).
11. T. Wada, A. Mochizuki, Y. Sato and M. Sekine, Tetrahedron Lett. 39, 7123 (1998) ; (b) Y. Hayakawa in "Comprehensive Organic Synthesis", Vol. 5, B. M. Trost and I. Flemming (eds), Pergamon Press, New york (1991), p. 601; (c) J. Stawinski in "Handbook of Organophosphorus Chemistry", R. Engel (ed), Dekker Publishers, New York (1992), p.377.
12. (a) P. J. Garegg, I. Smith, T. Redberg, J. Strowinski and R. Stromberg, Tetrahedron Lett. 27, 4051 (1986); (b) P. Westerduin, G. H. Veeneman, G. A. van der Marcel and J. H. van Boom, Tetrahedron Lett. 27, 6271 (1986).
13. (a) Methoden der Oragnischen Chemie, E. Muller (ed), Houben-Weyl, Georg Thieme, Stuttgart (1964) p.463; (b) M. S. Kharasch, R. A. Mosher and I. S. Bengelsdorf, J. Org. Chem. 25, 1000 (1960); (c) K. Troev and G. Borris, Phosphorus Sulfur 29, 129 (1987); (d) W. Gerrard, W. J. Green and R. A. Nutkins, J. Chem. Soc. 4067 (1952).
14. (a) D. A. Bafus, E. J. Gallegos and R. W. Kiser, J. Phys. Chem. 70, 2614 (1966); (b) J.-J. L. Fu and W. G. Bentrude, J. Am. Chem. Soc. 94, 7710 (1972).
15. (a) J. Krusic, W. Mahler and J. K. Kochi, J. Am. Chem. Soc. 94, 6033 (1972); (b) A. G. Davies, D. Griller and B. P. Roberts, J. Chem. Soc., Perkin Trans,. ll, 993 (1972) ; (c) K. J. Humphris and G. Scott, J. Chem. Soc., Perkin Trans. ll, 831 (1973); (d) C. Malav and J. Barrans, Tetrahedron Lett. 3077 (1975); (e) R. Burgada, Bull. Chim. Soc. (France) 407 (1975).
16. (a) G. O. Pierson and O. A. Runquist, J. Org. Chem. 34, 3654 (1969); (b) Y. Ito, M. Oda and Y. K. Kitahara, Tetrahedron Lett. 239 (1975); (c) C. H. Foster and G. A. Betchtold, J. Org. Chem. 40, 3743 (1975).
17. (a) C. B. Scott, J. Org. Chem. 22, 1118 (1957); (b) M. J. Borkin and D. B. Denney, Chem. and Ind. (London), 330 (1959).
18.(a) S. Trippett, J. Chem. Soc., Quart. Rev. 17, 406 (1963); (b) W. E. McEwen, A. Blade´-Font and C. A. Vanderwerf, J. Am. Chem. Soc. 84, 677 (1962); (c) R. M. Gerkin and B. Rickborn, J. Am. Chem. Soc. 89, 5850 (1967); (d) R. Huisgen and J. Wulff, Ber. 102, 1841 (1969); (e) W. J. Wadsworth, J. and W. D. Emmons, J. Am. Chem. Soc. 83, 6330 (1961); (f) R. M. Gerkin and B. Rickborn, J. Am. Chem. Soc. 89, 5850 (1967) and refs. cited therein; E. Zbiral, Monatsch 94, 78 (1963).
19. (a) A. R. Stiles, F. F. Rust and W. E. Vaughan, J. Am. Chem. Soc. 74, 3282 (1952); (b) "Chemistry of Phosphorus Compouds", Vol. 1, S. Patai (ed), Wiley and sons (New York) (1990).
20. A. N. Thakore, P. Pope and A. C. Ochlschlager, Tetrahedron 27, 2617 (1971).
21. (a) F. H.Westheimer, Chem. Rev. 81, 313 (1981); (b) A. C. Satterthwait and F. H.Westheimer, J. Am. Chem. Soc. 102, 4464 (1980).
22. (a) F. Ramirez, A. S. Gulati and C. P. Smith, J. Org. Chem. 33, 13 (1968); (b) G. W. Griffin, D. M. Gibson and K. Ishikawa, J. Chem. Soc., Chem. Comm. 595 (1975).
23). (a) W. G. Bentrude, J.-J. L. Fu and P. E. Rogers, J. Am. Chem. Soc. 95, 3625 (1973) and refs. cited therein; (b) D. A. Bafus, E. J. Gallegos and R. W. Kiser, J. Phys. Chem. 70, 2614 (1966); (c) C. Walling and R. Raboniwitz, J. Am. Chem. Soc. 81, 1243 (1959) ; (d) Y. Ogata and M. Yamashita, J. Chem. Soc., Perkin Trans. II, 730 (1976); (e) F. F. Rust, cf. Chem. Abstr. 50, 10124 (1956); (f) J. I. G. Cadogan and W. R. Foster, J. Chem. Soc. 3071 (1961); (g) M. M. Rouhut, H. A. Currier, J. M. Semsel and V. P. Wystrach, J. Org. Chem. 26, 5138 (1958); (h) Methoden Der Organischen Chemie (Houble-Weyl), Organische Phosphor Ferbindungen, Part 1, K. Sasse (ed), Georg Thieme, Stuttgart (1963); (i) D. Hellwinkel, Ber., 102, 528 (1969); (j) Free Radicals in Organic Chemistry, J. Fossey, D. Lefort and J. Sorba, Wiley and Sons (1995).
24. (a) A. R. Stiles , W. F. Vaughan and F. F. Rust, J. Am. Chem. Soc. 80, 714 (1958); (b) E. Maruszewska-Wieczorwska, J. Org. Chem. 26, 1886 (1958); (c) L. A. Hamilton and R. H. Williams, Chem. Abst. 50, 10317 (1961).
25. (a) J. G. Pritchard, Org. MassSpectrom. 3, 163 (1970); (b) P. Haake and P. S. Ossip, Tetrahedron 24, 565 (1969); (c) P. Haake, M. J. Frearson and C. E. Diebert, J. Org. Chem. 34, 788 (1969); (d) H. Budzikiewicz, C. Djerassi and D. H. Williams, Holden-Day (1967), ch. 26.
26. S. Munavalli, D. K. Rohrbaugh, G. W. Wagner, F. R. Long and H. D.
Durst, 2001 U.S. Army, ECBC Sci. Conf. on Chemical and Biological Defense
Research, Hunt Valley (MD), USA. 27. Ref. 25d, p.57.