Sixth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-6), 1-30 September
[A019]
The Selective Protection and Deprotection of Alcohols and Amines
Ibrahim Demirtas* and Bulent Buyukkidan
Department of Chemistry, University of Gaziosmanpasa, 60250, Tokat, Turkey
FAX: +90 356 2521585; E-mail: [email protected]
Abstract: The reactivity of nitrogen versus oxygen in the protection reactions were compaired. In principle, protection of aminophenols with trityls may take place on the oxygen. However, in this framework, we discuss the different reactivities of oxygen and nitrogen towards tritylchloride (TrCl) and 4,4’-dimethoxytritylchloride (DMTrCl) and come to the conclusion that the nitrogen is the preferred reaction. It is also discussed the selectively protection of oxygen with trityl.
Introduction
The problem of functional group incompatibility in the synthesis of complex organic structures has persisted since the pioneering research of our group on the synthesis of hydroxy and amino substituted compounds. One of our contributions to the developments of organic chemistry was the notion that an otherwise reactive functionel group could be temporarly rendered inert by appending a suitable protecting group1,2 which could then be later removed. Despite an intervening century of fabulous progress in the synthetic methodology3,4, the proliferation of protecting groups is a tacit acknowledgement that selectivity in functional group transformations remain a central and unsolved problem in organic synthesis5.
In this work, we analyse the oxygen reactivity versus the nitrogen reactivity in an ambident nucleophile of o-aminophenol (9) m-aminophenol (1) and p-aminophenol (12). As a preliminary work, we found that the nitrogen atom is the reactive centre and protected easily compairing with the oxygen atom as shown in Scheme-1, Scheme-2 and Scheme-3.
The proton and carbon NMR studies of the compounds 2, 3, 4, 5, 6, 7 and 8 indicated the formation of the assigned structures (Scheme-1). The infrared spectra of the compound 5 clearly show the highest frequency band due to the O-H and N-H stretching vibration at around 3500-3000 cm-1 (Figure 1).
 |
Figure 1: The infrared spectra of compound 5

Scheme-1
It is noteworthy that NH-DMTr, unlike O-Tr, in compound 3 (Scheme-1) and compound 14 (Scheme-3) led to its decomposition using the silica column during the purification, but not decomposed in alumina column.
The Scheme-2 show the protection of o-aminophenol (9) with TrCl and DMTrCl. The nitrogen was protected with TrCl to give compound 11 and with DMTrCl to give compound 10. However, the further attempt for protection of oxygen was unsuccesful due to the steric effect. The structures of compound 9 and 11 were assigned using the proton and carbon NMR.
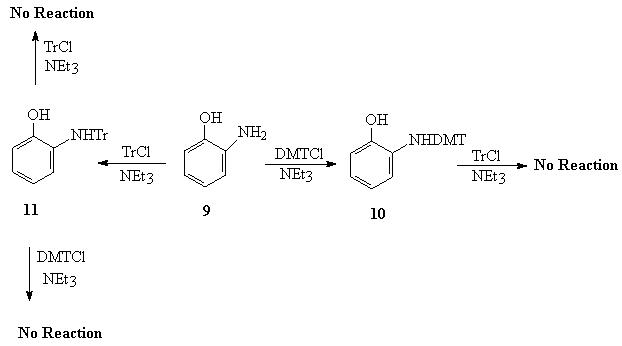
Scheme-2
According to Scheme-3, the nitrogen and oxygen of p-aminophenol was selectively protected with TrCl and DMTrCl to give compound 15 and 16. The proton and carbon NMR studies of the compound 15 and 16 indicated the formation of the assigned structures.
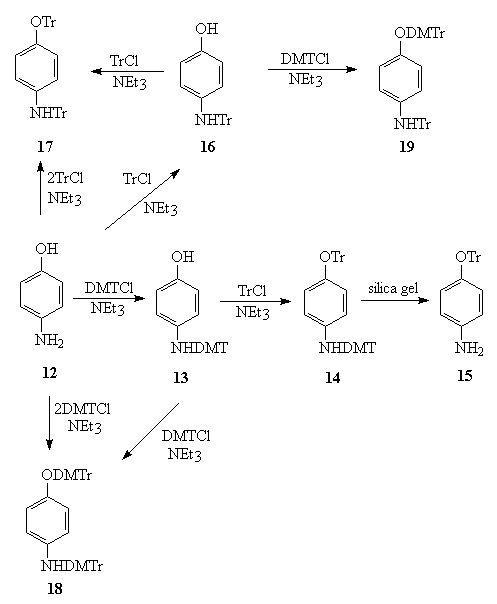
Scheme-3
The compounds 13, 14, 17, 18 and 19 were also identified using the carbon and proton NMR and infrared.
Conclusion
The results described in this paper lead us to recommend the wider use of TrCl and DMTrCl protecting groups for primary hydroxyl and amino groups. The DMTrCl group is not only selective because of its stability in air, but also relative ease of removal from protected amino group.
Acknowledgements
I thank the Department of Chemistry, Gaziosmanpasa University (Grant Nr:2001/29) for financial support.
References
1. I. Demirtas, Fift International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5), 1-30 September 2001; M. C. Lopez, W. Clegg, I. Demirtas, M. R. J. Elsegood, and H. Maskill, J. Chem. Soc. Perkin Transactions 2, 2000, 1, 85-92.
2. C. Bleasdale, S. B. Ellwood and B. T. Golding, J. Chem. Soc. Perkin Transactions 1, 1990, 803-805; A. P. Henderson, J. Riseborough, C. Bleasdale, W. Clegg, M. R. J. Elsegood and B. T. Golding, J. Chem. Soc. Perkin Transactions 1, 1997, 3407-3413.
3. M. C. Lopez, J. C. Martinez, I. Demirtas, H Maskill, and E. Stix, Org. React., 1997, 30, 71-77.
4. M. C. Lopez, I Demirtas and H. Maskill, J. Chem. Soc. Perkin Transactions 2., 1748-1752.
5. M. C. Lopez, W. Clegg, I. Demirtas, M. R. J. Elsegood, J. Haider, H. Maskill, and P.C. Miatt, J. Chem. Soc. Perkin Transactions 2, 1742-1747.