Marcin Lukasiewicz, Dariusz Bogdal
Department of Polymer Science, Politechnika Krakowska,
ul. Warszawska 24 31-155 Krakow, Poland
e-mail: [email protected]
| Abstract | Keywords | Introduction | Experimental | Literature |
Abstract
The employing of tetravalent chromium oxide (Magtrieve™) in
oxidation of some simple alcohols and hydrocarbons under microwave
irradiation were shown. Many advantages of such new oxidation protocol
comparing to conventional heating and using another oxidants were pointed
out.
Keywords
Magtrieve™; microwaves; oxidation
Introduction
Microwave processing of materials offers distinct advantages over
conventional heating in some applications [1]. The
energy of the microwave field can be dissipated directly into the desired
media, without the convection and conduction associated with conventional
heating [2]. The
introduction of microwave energy into a chemical reaction which has at
least one component which is capable of coupling strongly with microwaves
can lead to much higher heating rates than those which are achieved
conventionally [3].
Additionally the reactants do not interact equally with the commonly used
microwave frequency for dielectric heating and consequently selective
heating can be achieve [4]. Since
early eighties there is a lot of reports on conducting organic
transformation in the presence of microwave fields. The oxidation reaction
are also a part of these investigation (using permanganate, dichromate,
hydrogen peroxide, persulphates and others oxidant [5]). Magtrieve™ is DuPont's trademark for the oxidant
based on tetravalent chromium dioxide (CrO2) [6]. Scheme 1
Scheme 1
In our research on oxidation
processes, we chose Magtrieve™ as an oxidant, because it has been proven
to be a useful oxidant in some reactions including the oxidation of
alcohols [7]. Magtrieve™ as an oxidant is very well suited reagent
for microwave synthesis, because as an ionic and magnetically retrievable
material, it carries a benefit of so efficient converting of
electromagnetic energy into heat according to dielectric heating
mechanism. Scheme
2
Scheme
2
The investigation were focused on the oxidation of
alcohols to corresponding carbonyl compounds (illustrated on Scheme 1 by
example of 2-octanol) and side-chain hydrocarbons to equivalent ketons
(illustrated on Scheme 2 by example of fluorene).
Experimental
All experiments were carried out in multimode and monomode
microwave reactors (Plazmatronika Poland). The reaction procedure
involves simple mixing of 1g of the substrate (alcohol or hydrocarbon), 5g
of Magtrieve™ and 20mL of toluene. The heterogenic mixture were placed in
the reactor and irradiated during specific period of time (see Table 1)
under reflux. The power of applying microwaves were set in order to keep
the reaction mixture boiling. After completing of the reaction the oxidant
were separated by the magnet and the solvent were evaporated resulting the
crude product which was purified by distillation or crystallization.
Result and Discusion
As a result we have obtained a number of desired
carbonyl product showed in the table 1 and 2.
Table 1. Microwave oxidation of alcohols by Magtrieve™
Substrate
Product
Yield [%]
Time [min]
![]()
![]()
99
25
![]()
![]()
73
30
![]()
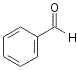
96
5
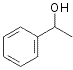
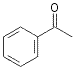
65
20
![]()
![]()
85
30
The satisfactory yields of conducted reaction shows that Magtrieve™ is an
useful microwave-working oxidant. Because its ionic structure and magnetic
properties Magtrieve™ is strongly coupled with microwave irradiation.
Table 2. Microwave side-chain oxidation of hydrocarbons by Magtrieve™
Substrate
Product
Yield [%]
Time [min]
![]()
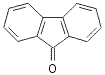
54
90
![]()
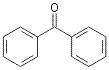
25
90
![]()
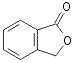
65
70
![]()
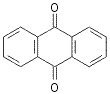
86
60
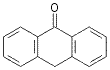

96
45
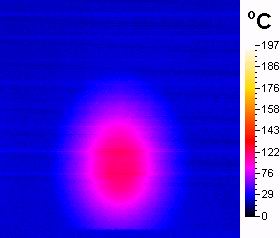
The experiment
of irradiating of pure oxidant showed dramatic increase in temperature of
the material up to 370�C in 2 minutes (Scheme 3) what was mesured by
infrared camera. The addition of nonpolar solvent (toluene) prevents the
substrate to keep of burning (what was observed in additional experiment
where only the liquid substrat i. e. alcohol and oxidant were irradiated)
but the temperature of the reaction mixture after 2 minutes of irradiation
reaches the boiling point of the solvent and the surface of the oxidant
has about 140�C (Scheme 4).
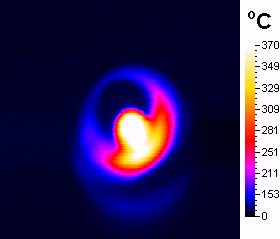
Scheme
3.Pure Magtrieve™ after
2minutes of irradiation
Scheme
4.Reaction mixture after
2minutes of
irradiation
In conclusion we
can say that, however we have used a transition metal (chromium) oxidant,
the describing procedure is environmental friendly because of short
reaction time, easy set-up and separation of oxidant. Additionally the
recycling of the oxidant (described in literature) ranks the described
method of oxidation as a powerful and "green" tool in modern organic
synthesis.
Literature
[1]. P. Lidstroem, J. Tierney, B. Wathey, J.
Westman; Tetrahedron; 2001, 57, 9225
[2]. C. Gabriel, S.
Gabriel, E. Grant, B. Halstead, D.P. Mingos; Chem. Soc. Rev.; 1998,
27,213
[3]. H. M. Kingston, J. S. Haswell; "Microwave-
Enhanced Chemistry. Fundamentals, Sample Preparation and Applications";
American Chemical Society, 1997
[4]. L. Perreux, A.
Loupy; Tetrahedron, 2001, 9199
[5]. a)D. Bogdal, M.
Lukasiewicz; Synlett, 2000, 1, 143 b) R. Varma, R. Saini, H. Meshram;
Tetrahedron Lett.; 1997, 38, 6225 c) R. Varma, R. Saini, R. Dahiya;
Tetrahedron Lett, 1997, 38, 7823 d) R. Varma, R. Saini; Tetrahedron Lett;
1998, 39, 1481
[6]. a) US Pat. 4 524 008(1985) b) US Pat
3 278 263(1966)
[7].R. Lee, D. Donald; Tetrahedron Lett;
1997, 22, 3857