 |
microwave enhanced radical aromatic substitution:Chlorination of anthracene |
 |
7th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-7), http://www.mdpi.net/ecsoc-7, 1-30 November 2003
[E002]
 |
microwave enhanced radical aromatic substitution:Chlorination of anthracene |
 |
Julio A. Seijas,* M. Pilar Vázquez-Tato,* Raquel Carballido-Reboredo
Departamento de Química Orgánica. Facultad de Ciencias. Universidad de Santiago de Compostela. Campus de Lugo. Aptdo. 280. 27080-Lugo. Spain
Irradiation with microwaves is a well known method to speed up reactions, with the added value of usually avoiding the use of solvents with environmental benefits. Our group is interested in searching new applications of this methodology to promote organic reactions. Amongst the several methods available to chlorinate aromatics hydrocarbons, the use of copper salts (chloride or bromide) is a good yield rendering methodology and easier than chlorine gas. Halogenation with cupric chloride was presented as polar electrophilic aromatic substitution1or ligand transfer2 although actually it is considered to be a radical aromatic substitution:3, 4
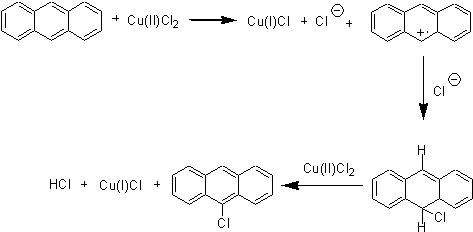
Conventional syntheses were reported for 9-chloroanthracene (anthracene:CuCl2 1:2, CCl4, reflux 24h, 75-80% yield)1 and for 9,10-dichloroanthracene (anthracene:CuCl2 1:4.5, 3-chlorotoluene, 125ºC, 1.5h, 86-97% yield),5 both reactions employed solvents with a high environmental impact. This procedure for chlorination has been extensively studied, and although several assays have been carried out in the absence of solvent these have not been successful. This prompted us to study this reaction under microwave irradiation.
Our preliminary experiments with anthracene and cupric chloride in solid state, did not show reaction (5-10 minutes irradiation), this could be attributed to the low absorption of microwaves by a molecule with a low polarity as anthracene. Thus we decided to add a component with a high coupling with microwaves, we decided to use graphite since it has a high absorption towards microwaves. The addition of this component, led to a great enhancement of the reaction. Its behavior as susceptor gives rise to hot spots and is this more than its ability to adsorb organic compounds what enhances the reaction, since it had been reported4 that CuCl2/graphite/ClPh with conventional heating was not a practical method to chlorinate aromatic hydrocarbons.
We have carried out several experiments and found that a 10% w/w graphite/anhydrous cupric chloride was the best ratio, higher ratios of graphite led the reaction to be out of control, with incandescence and sparkling in the reaction flask, even with melting of the Pyrex glass, in fact we had to carry out our reactions on porcelain flask adapted with an air condenser. Irradiation in high ratios of graphite/CuCl2 could lead to reduction of the copper salt to Cu(0). Another important factor is the ratio anthracene/CuCl2, we have tried several ratios from 1:1 to 1:5 observing that under irradiation with microwaves (80% of 1000W total output, ETHOS D-Millestone, 1-3 minutes) only with 1:5 ratio a pure product (checked by NMR) was obtained identified as 9,10-dichloroanthracene in a quantitative yield. Under other reaction conditions were obtained mixtures of anthracene/9-chloroanthracene/9,10-dichloroanthracene.

This method provides an easy way to prepare 9,10-dichloroanthracene, which has a wide use in fluorescence applications, free of the use of environmentally harmful solvents.
Acknowledgements
XUNTA DE GALICIA for financial support: PGIDT01PXI26203PR and PR405 A 098/59-0
1.- Nonhebel, D. C. Organic
Synthesis Coll. Vol. 5, 206
2.- Mosnaim, D.;
Nonhebel, D. C. Tetrahedron 1969, 25, 1951-5.
3.-
Tanimoto, I.; Kushioka, K.; Kitagawa, T.; Maruyama, K. Bull. Chem. Soc.
Jpn. 1979, 52, 3586-3591.
4- Kodomari, K.; Satoh, H.;
Yoshitomi, S. J. Org. Chem. 1988, 53,
2093-2094
5.-
Kauffman, J. M., Synthesis 2001, 197-199.
|
|