

CHEMICAL SYNTHESES AND
TECHNOLOGIES
FOR THE SUSTAINABLE DEVELOPMENT III.
Pavel PAZDERA and Jan ŠIMBERA


Abstract
Environmental friendly synthesis of butane-1,2,3,4-tetracarboxylic acid starting
from succinic anhydride is described. Major synthetic step,
i. e. coupling between bromosuccinic anhydride and succinic anhydride was
realized under ultrasonochemical solid – liquid phase transpher catalysis (US
s-l PTC) conditions.
Butane-1,2,3,4-tetracarboxylic acid is produced as broad applicable product in relative sizable volumes2. It is a very good formaldehyde-free durable press finishing agent, which has high reaction activity and no irritant odor. In addition, this carboxylic acid can be used in manufacturing polyimide materials which have heat-proof, acid-proof and hydrocarbon-proof properties, used in production of functional polymer materials such as photosensitive materials, biomedical materials and functional polymeric membrane materials. Furthermore, the derivatives and polymers of butane-1,2,3,4-tetracarboxylic acid are excellent electricity insulator, and are widely used in the producing of electricity insulative rings, wire enamels and electricity insulator materials.
Common processes of butane-1,2,3,4-tetracarboxylic acid fabrication3 copy the three steps synthetic scheme (Scheme 1).
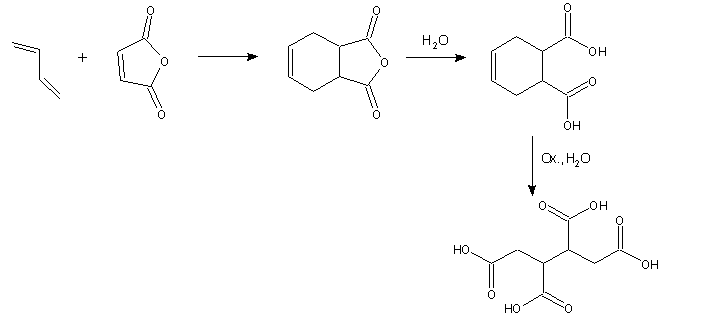
Scheme 1. Common synthetic way to butane-1,2,3,4-tetracarboxylic acid production
3a,4,7,7a-Tetrahydro-2-benzofuran-1,3-dione is prepared by Diels-Alder
reaction of butane-1,3-diene and maleic anhydride in the first step. Second
synthetic step is its hydrolysis to cyclohex-4-ene-1,2-dicarboxylic acid and
synthesis is finished by oxidative hydrolysis of this intermediate product. This
last step is not environmental friendly because it makes using of strong
oxidizing reagents (hydrogen peroxide, nitric acid, potassium permanganate,
ozone) and heavy metal compounds (copper, vanadium, tungsten). Further problem
is also contamination of final butane-1,2,3,4-tetracarboxylic acid by these
heavy metal compounds and an occasion its refinement. On the other hand, the
overall process
of butane-1,2,3,4-tetracarboxylic acid production is started from relatively
moderate petrochemical products.
Now we yield new different way for the butane-1,2,3,4-tetracarboxylic acid synthesis according to the Chemical Syntheses and Technologies for the Sustainable Development.
The synthesis of butane-1,2,3,4-tetracarboxylic acid followed the next scheme, all process is started from succinic anhydride as a relatively moderate petrochemical product without the presence of heavy metals or further hazardous reagents:
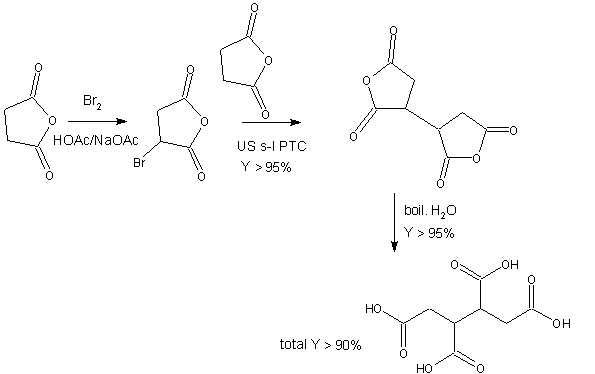
Scheme 2. Synthetic way to butane-1,2,3,4-tetracarboxylic acid according to the Sustainable Chemistry
The first step of the synthesis is brominating of succinic anhydride into acetic acid solution in the presence of sodium acetate as the base at room temperature. The bromine may be generated from bromide anion in situ by electrolysis. This spontaneous brominating reaction is known generally and gives bromosuccinic anhydride quantitatively. This product should not to be purified. After evaporation of liquid (acetic acid can be recycled) follow the next step realized in dioxane solution.
The second step presents the sonochemical s-l PTC4,5 coupling between bromosuccinic anhydride and the carbanion generated from succinic anhydride by an action of equimolar mixture of calcium carbonate – calcium oxide in the Cetrimide presence at temperature 30-40 °C. This coupling gives tetrahydro-3,3'-bifuran-2,2',5,5'-tetrone (butane-1,2,3,4-tetracarboxylic acid bis-anhydride) in the course ca 60 min in very good yield. The bis-anhydride should not to be purified. Silica is added ending of this reaction and a solid complex is filtered off (bromides may be regenerated to bromine by electrolysis).
After dioxane evaporation (recycling of dioxane is necessary) is the solid
pure product mixed with water and a hydrolysis proceeds
at 90-100°C. The final product is separated by suction after cooling. The last
step is quantitative, purity and quality of the product after drying
is very good.