8th International Electronic Conference on Synthetic Organic Chemistry. ECSOC-8. 1-30 November 2004. http://www.lugo.usc.es/~qoseijas/ECSOC-8/
Synthesis of Sulfapyridine metabolites
Petr Knesl , and Ulrich Jordis
Institute of Applied Synthetic Chemistry, Vienna University of Technology
Getreidemarkt 9, 1060 Vienna, Austria, [email protected]
______________________________________________________________________________________________________________________
Abstract:
Sulfapyridine (SP) is an antibacterial compound that is used as such and also the active metabolite of sulfosalzazine (SASP) which shows antiphlogistic, antibacterial and immunomodulatoric properties. Sulfapyridine is also used to help control dermatitis herpetiformis (Duhring's disease), a skin problem. It has been reported, that sulfapyridine is metabolized to 5-hydroxysulfapyridine but no synthesis of this proposed metabolite has been published.We will report on the synthesis of thia and other potential metabolites of sulfapyridine.
______________________________________________________________________________________________________________________
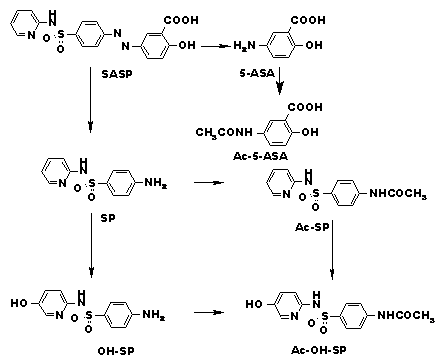
Sulfapyridine (SP) and sulfasalazine (SASP) are used for the treatment of Crohn`s disease, rheumatoid arthritis and ulcerative colitis. When SASP is given orally it is partly absorbed unchanged and partly cleaved to aminosalicilic acid (5-ASA) and SP by bacterial azo reductase1. SP is the therapeutically active moiety of SASP and undergoes further acetylation, hydroxylation and subsequent glucoronidation2,3. The main metabolites were reported to be the N-acetylsulfapyridin (Ac-SP) and 5-hydroxy-sulfapyridine (OH-SP). Formation of Ac-SP and OH-SP are under genetic control and the proportion of acetylated and hydroxylated SP in serum and urine depends on the genetically determined acetylation and hydroxylation phenotypes of the patients4
The main metabolic pathway is depicted in Scheme 1.
Scheme 1: Metabolism of SASP

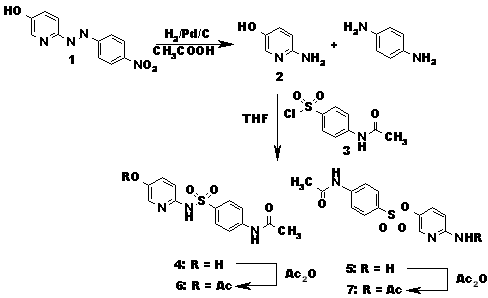
To the best of our knowledge no references describing the synthesis of SASP metabolites have been reported, although several papers regarding analytical separation4,5 and metabolism1,2,3 including OH-SP and Ac-OH-SP were published. As starting material for our synthesis we used 6-(4-nitro-phenylazo)-pyridin-3-ol6 1 which was reduced with H2/Pd to the higly unstable 5-hydroxy-2-aminopyridin 2 and immediately treated with N-Acetyl-sulfonylchlorid 3 to give a mixture of at least four products that were separated by LC (Scheme 2). From the isomers 4 and 5 the acetylated compounds 6 and 7 were prepared and the structures of 6 and 7 assigned by NMR thus allowing also to distinguish between 4 and 5.
Scheme 2: Synthesis of AC-OH-SP (4)
As none of these compounds showed LC/MS behavior similar to the expected metabolites in the urine of persons treated with SP, two additional known7,8 oxygenated SP derivatives N-[4-(1-Oxy-pyridin-2-ylsulfamoyl)-phenyl]-acetamide 9 and N-[4-(Hydroxy-pyridin-2-yl-sulfamoyl)-phenyl]-acetamide 10 with the same mass as Ac-OH-SP were synthesized (Scheme 3). N-acetylsulfapyridine 8 was oxidized to afford a mixture of both products9 that were separated by LC. Neither 9 nor 10 could be detected in the urine of persons treated with SP.
Scheme 3: Synthesis of potential metabolites

Conclusion:
We have prepared and characterized potential SP metabolites. Preliminary results raise some doubts to the published metabolism of SP. The compounds await further biochemical investigation.
References:
1. Sjoequist, B.; Ahnfelt, N.O.; Stig, D.R.; Fjellner,G.; Hatsuoka, M.; Ljungstedt-paahlman, I.; Chem.Biomed.Res., Pharm.Med.Prod. 6, 1991, 491
2. Zheng, W.; Winter, S.M.; Mayersohn, M.; Bishop, J.B.; Sipes, I.G. Drug Metabolism and Disposition 21, 1993, 1091
3. Sjoequist, B.; Ahnfelt, N.O.; Stig, D.R.; Fjellner,G.; Hatsuoka, M.; Ljungstedt - paahlman, I.; Chem.Biomed.Res., Pharm.Med.Prod. 6, 1991, 425
4. Rona, K.; Winkler, V.; Riesz, T.; Gachalyi, B. Chromatographia 26, 1988, 393
5. Vree, T.B.; Martea, M.; Lewin, L.M. Journal of Chromatography 534, 1990, 214
6. Moore, J.A.; Marascia, F.J. J.Am.Chem.Soc. 81, 1959, 6049
7. Bobranski, B.; Mordarski, M.; Pelczarska, A.; Pomorski, J. Pol. Arch.Immunol.Ther.Exp. 16, 1968, 804
8. Bobranski, B.; Pomorski, J. Bull.Acad.Polon.Sci. 7, 1959, 203
9. Childress, S.J.; Scudi, J.V J.Org.Chem. 23, 1958, 67