Scheme 1
[A001]
J. Domingo Sánchez, Carmen Avendaño and
J. Carlos Menéndez
Departamento de Química Orgánica y Farmacéutica, Facultad de Farmacia.
Universidad Complutense, 28040 Madrid, Spain.
E-mail:[email protected]
Introduction
N-Protection is a very important aspect of indole chemistry because of the poor stability of indole under a variety of conditions, specially acidic ones, and also because N-protection allows, and often directs, the lithiation of the heterocyclic nucleus. Due to the high basicity and nucleophilicity of the indole five-membered ring, protection of the C2-3 bond can also be sometimes desirable. The most commonly employed indole N-protecting groups are arylsufonyl derivatives (e.g. tosyl), carbamates (e.g. BOC), trialkylsilyl groups (e.g. triisopropylsilyl), N,O-acetals (e.g. SEM) and some alkyl groups (e.g. benzyl) [1]. Protecting groups for the indole C2-3 bond are currently very scarce, and based on the use of expensive starting materials [2].
Pivaloyl would be a very interesting protecting group for indole because, due to steric reasons, it protects both the N-1 and C-2 indole positions. For instance, it has been used to direct intramolecular Friedel-Crafts acylations of indole-3-propionic acid derivatives to C-4 rather than to the electronically more favourable C-2 [3]. However, pivaloyl is notoriuosly difficult to remove. Although deprotection of pivaloylindoles has occasionally been achieved by use of alkoxides, the generality of this method has not been established because it has been studied only in a few specific cases, and the yields found are variable and sometimes very poor. For instance, sodium methoxide gave only 19% yield in the deprotection of a N-pivaloylcyclohepta[cd]indole derivative [4], although it had worked very well on a closely related cyclohexa[cd]indole system [5].
Due to the potential advantages of
pivaloyl as a protecting group for indole, a general deprotection method
of pivalolyindoles is desirable. Is this context, we report here our findings
the use of LDA to achieve this transformation.
During the course of our studies on
the synthesis of compounds related to the natural multi-drug resistance
inhibitor N-methylwelwitindolinone C isothiocyanate (welwistatin,
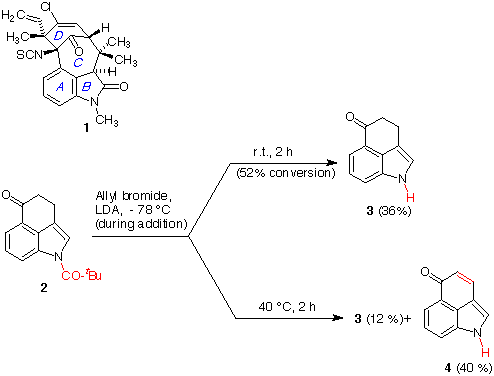
compound 1) [6,7] we examined the alkylation of tricyclic ketone 2
[3,5] with allyl bromide in the presence of LDA as a base at - 78 °C,
followed by 2 h qat room temperature. The only observed product (together
with unreacted starting material) was compound 3, from depivaloylation
of 2. Harsher conditions (2 h at 40 °C) led to a mixture
of 3 and 4, presumably arising from hydroxylation of the anion
derived from 3 by traces of oxygen, followed by elimination.

Scheme 1
The observations summarized in Scheme 1 led us to consider the possibility of employing LDA for the deprotection of pivaloylindoles. After some experimentation, we found that treatment of pivaloylindole itself with 2 equivalents of LDA in THF at 40-45 °C led to its quantitative deprotection. In order to verify the generality of the method, several variously substituted pivaloylindole derivatives 5 were prepared by treatment of the corresponding indoles with sodium hydride followed by addition of the suitable alkyl halide. The results obtained in the reaction of these compounds with LDA to give the unprotected indoles 6 are summarized in Scheme 2 and Table 1.
The reactions carried out on a number
of indoles substituted at the 3, 4, 5 and 7 positions (entries 2-7) proceeded
in excellent yields. A variety of functional groups like aldehyde (entry
3), ester (entries 6 and 7) and ether (entry 8) were tolerated without any
noticeable decomposition, and a carboxylic group in a side chain at C-3 caused
only a slight decrease in yield (entry 9), in spite of its reactivity towards
LDA. On the other hand, and because of steric interference, 2-substituted
and 7-substituted indoles were less reactive and thus the reactions starting
from 7-methyl-1-pivaloylindole (entries 4 and 5) and 2-phenyl-1-pivaolylindole
(entries 10 and 11) required 40 h and 90 h to achieve completion, respectively,
although the yields were
again excellent.
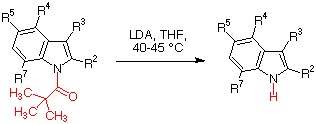
Scheme 2
Table 1
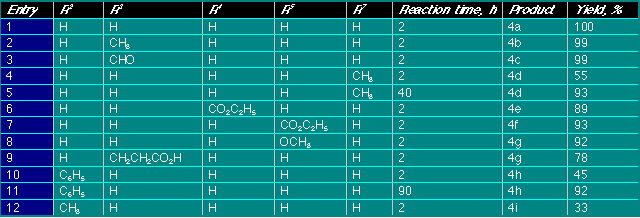
The reaction starting from 2-methyl-1-pivaloylindole,
together with the expected compound 6i, gave 8 as the
major product, presumably by assisted metallation to 7 followed by
intramolecular transfer of the pivaloyl group. Intermolecular pivaloyl transfer
seems to be excluded by the very different yields obtained for 6i
and 8.
Scheme 3
We also studied the deprotection of
several more complex heterocyclic systems containing N-pivaloylated
indole subunits, including carbazole 9, a pyridocarbazole derivative
11 and beta-carboline 13. As shown in Scheme 4, these reactions
also proceeded in good to excellent yields.
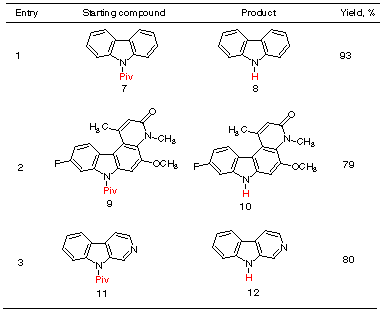
Scheme 4
Acknowledgment
We thank CICYT for financial support of this research through grant SAF
2000-0130.
References
(1) (a) Kocienski, P. J. Protecting
Groups (3rd Ed.). Georg Thieme Verlag, 2004.
(b) Greene, T. W.; Wuts, P. G. M. Protective
Groups in Organic Synthesis (3rd Ed.), pp. 615-631. Wiley-Interscience, 1999.
(2) Baran, P. S.; Guerrero, C. A.; Corey, E. J. Org. Lett. 2003, 5, 1999-2001.
(3) Teranishi, K.; Hayashi, S.; Nakatsuka, S.; Goto, T. Tetrahedron Lett. 1994, 35, 8173-8176.
(4) Horwell, D. C.; McKiernan, M. J.; Osborne, S. Tetrahedron Lett. 1998, 39, 8729-8732.
(5) Teranishi, K.; Hayashi, S.; Nakatsuka, S.; Goto, T. Synthesis 1995, 506-508.
(6) (a) Stratmann, K.; Moore, R. E.;
Bonjouklian, R.; Deeter, J. B.; Patterson, G. M. L.; Shaffer, S.; Smith,
C. D.; Smitka, T. A. J. Am. Chem. Soc. 1994, 116, 9935-9942.
(b) Smith, C. D.; Zilfou, J. T.; Stratmann,
K.; Patterson, G. M. L.; Moore, R. E. Mol. Pharmacol. 1995,
47, 241-247.
(7) For a review of synthetic work
on welwitindolinones and related areas, see: Avendaño, C.; Menéndez,
J. C. Curr. Org. Synth. 2004, 1, 65-82.