Introduction
Pyronines B (PB) and Y (PY) are cytotoxic dyes used in the Unna Pappenheim stain for ribonucleic acid. They provide simplified models of the xanthene skeleton of rhodamines, which are also used in the labeling of proteins and cell organelles. The interaction between these dyes and organized media can be studied using the complexation of these pyronines with cyclodextrins which are well known model systems due to their ability to form inclusion complexes. The specific noncovalent interactions between cyclodextrins and guest molecules resemble those controlling molecular recognition phenomena in biological systems [1].
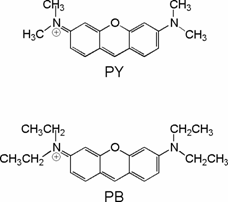

Figure 1. Structures of pyronines Y and B and β-cyclodextrin.
In this work we study the complexation of PY and PB with β-cyclodextrin (CD) using, together with UV-Vis absorption, the more sensible techniques of steady-state and time resolved fluorescence. The aim of the study is to characterize the photophysical properties of these pyronines when included in the hydrophobic cavity of CD in order to discuss the guest-host interactions involved in the formation of the complexes.
Experimental section and data analysis
These pyronines are not chemically stable because they hydrolyze in water, and more easily in basic media to the corresponding xanthydrol [2,3]. We have used acid and freshly prepared stocks to minimize the hydrolysis. Also the concentrations used were low enough to avoid aggregation of the pyronines in aqueous solution [2,4,5]. The stock solutions of PY (Sigma, 56%) and PB (Aldrich,57%) were prepared by dissolving the commercial product in water and immediately filtering to separate the impurities, which are not soluble in water [6]. The samples for the titrations with CD were prepared by addition of different volumes of a CD stock solution (0.011 M) to a fixed pyronine concentration, being always an excess of CD with respect to pyronine.
Absorption spectra were recorded in a Varian-Cary 300 spectrometer. Both steady state and time resolved fluorescence measurements were performed with an Edinburgh Instruments F900 spectrofluorimeter. All measurements were performed at a constant temperature of 20ºC.
The series of absorption and emission spectra recorded in the titrations with CD were analyzed using a self-developed procedure based on Principal Components Analysis and Global Analysis (PCGA) described elsewhere [7]. This method yields first the number of spectral components responsible for the observed variations in the series of absorption and emission spectra. This is the basis to draw up a theoretical model which is then used in a nonlinear least-squares global analysis of the spectra to obtain the physicochemical parameters involved and the individual spectra of the components.
Individual fits of fluorescence decays were performed with the software package from Edinburgh Instruments. We also applied a “target” global analysis to the series of decays with different CD concentrations. This analysis fits two coupled theoretical models, one for the time dependence and another one for the concentration dependence of the fluorescence intensity.
Rhodamine B was used as fluorescence standard for the determination of absolute fluorescence quantum yields (Φ) [8]. Radiative deactivation rate constants (kr) and nonradiative deactivation rate constants (knr) were obtained from the experimental values of the fluorescence quantum yields and the fluorescence lifetimes.
Results and discussion
Titration experiments were performed in order to investigate the complexation ability of the pyronines under study with CD. Figure 2 shows the series of absorption and emission spectra of PY in aqueous solutions with different CD concentrations. The absorption spectrum shows slight red shift and decrease of the molar absorptivity as the CD concentration is increased, with a clear isosbestic point indicating the presence of two species in equilibrium. The emission spectrum of PY shifts also to lower energies and decreases significantly in intensity when increasing the concentration of CD. The absorption and emission spectra of PB show analogous variations with the addition of CD, but they occur at smaller CD concentrations. This is shown in figure 3, where the molar absorptivities and the fluorescence intensities at the absorption and emission maxima, respectively, are plotted against CD concentration.
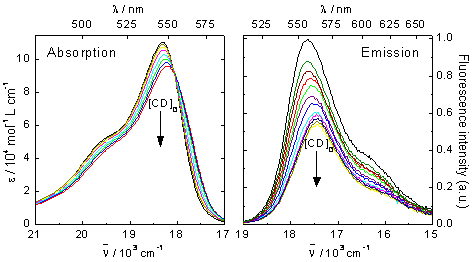
Figure 2. Series of absorption and corrected emission spectra of PY in aqueous solution
in the presence of different concentrations of CD, from 0 to 0.011 M. λexc = 515 nm.
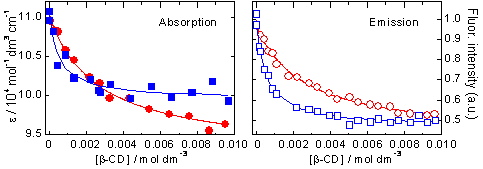
Figure 3. Plots of molar absorptivities and fluorescence intensities versus CD concentration for PY (circles in red)
and PB (squares in blue) at their respective absorption and emission maxima. The lines are the fitted curves.
Principal Components Analysis of the series of absorption and emission spectra indicate that, both for PY and for PB, only two components are necessary to explain the systematic variations of the spectra with the concentration of CD. This means that two chemical species are contributing to the observed absorption and emission spectra. On the basis of this result, the simplest model to consider is a 1:1 complexation equilibrium in the ground state, defined by an association equilibrium constant K with two species involved, the free pyronine P and the complex P:CD, since free CD do not absorb or emit UV-visible light. The observed absorption spectra are linear combinations of the individual absorption spectra of species P and P:CD, which are different due to the variation of the photophysical properties of the pyronines when included into the CD cavity. The same is true for the emission spectra, which are linear combinations of the emission spectra of the excited species P* and P:CD*. Since the lifetimes of the excited species are very short, no association/dissociation processes can take place in the excited state, so that the proposed mechanism is the following:
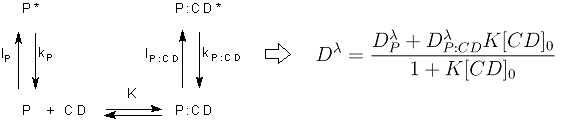
On the basis of this mechanism and taking into account that CD is in excess, a function can be deduced that relates the molar absorptivity or the fluorescence intensity at a certain wavelength (Dλ) with the total CD concentration (see equation above). This function was used as fit function in the global analysis of the series of absorption and emission spectra of the two pyronines with CD. The agreement between the fitted curves and the experimental data and the random residuals indicate good fits and validate the model proposed (see figure 3). The results of the fits are the values of the association equilibrium constants (table 1) and the individual spectra of the free pyronines and P:CD complexes (figure 4). There is a very good agreement between the values of K obtained from absorption and from emission data for each pyronine, result that supports the mechanism proposed. The association equilibrium constant of PB is about 5 fives larger than that of PY, indicating the higher stability of the complex PB:CD in comparison with PY:CD.
|
K / 103 mol-1 dm3 |
PY |
PB |
|
Absorption spectra |
0.39 ± 0.05 |
2.0 ± 0.4 |
|
Emission spectra |
0.40 ± 0.04 |
2.1 ± 0.2 |
|
Fluorescence decays |
0.36 ± 0.03 |
2.0 ± 0.1 |
Table 1. Results of the association equilibrium constants of PY and PB with CD obtained by global analysis of the series
of absorption and emission spectra, and by target global analysis of the series of fluorescence decays.
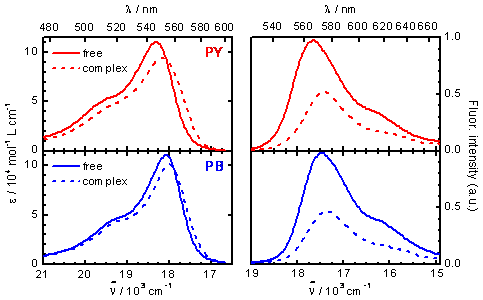
Figure 4. Absorption (left) and emission (right) spectra of PY (red) and PB (blue) as free molecules in aqueous solution (solid lines) and complexed by CD (dashed lines), as obtained by global analysis of the series of absorption and emission spectra.
Fluorescence decays of PY and PB in aqueous solutions with different CD concentrations were measured. The decays without CD yield the fluorescence lifetimes of the free pyronines given in Table 2. When adding CD, an additional shorter lifetime is obtained both for PY and for PB. This is assigned to the complex P:CD, whose contribution to the decay (preexponential factor) increases as the CD concentration is increased. In order to obtain good values for the complex lifetimes and to check the model proposed, “target” global analysis of the series of decays were performed with the model given by the following equation:
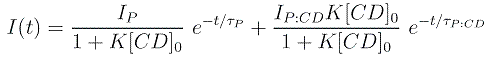
where the preexponential factors depend on the CD concentration in the same way as the ground-state concentrations of P and P:CD and the exponential functions are characterized by the fluorescence lifetimes of the excited species, P* and P:CD*. This equation fits satisfactorily all decays and the values obtained for the equilibrium constants are in good agreement with those determined from absorption and emission spectra (see Table 1). Also accurate values for the lifetimes of the complexes are obtained, as given in Table 2.
|
|
τ / ns |
Φ |
kr / 109 s-1 |
knr / 109 s-1 |
|
PY |
1.76 ± 0.01 |
0.47 |
0.27 |
0.30 |
|
PY:CD |
1.08 ± 0.03 |
0.27 |
0.25 |
0.68 |
|
PB |
1.17 ± 0.01 |
0.36 |
0.31 |
0.56 |
|
PB:CD |
0.50 ± 0.05 |
0.19 |
0.38 |
1.6 |
Table 2. Fluorescence lifetimes, fluorescence quantum yields, radiative deactivation rate constants and nonradiative deactivation rate constants of the free pyronines PY and PB and their inclusion complexes with CD, PY:CD and PB:CD.
These results indicate that the fluorescence lifetimes of the two pyronines decrease significantly when these molecules are included into the CD cavity. An analogous effect is observed in the fluorescence quantum yields (Table 2), that can be calculated from the individual emission spectra of the free pyronines and the complexes P:CD in Figure 4, using Rhodamine B as reference. For the two pyronines the fluorescence quantum yield decreases to about a half in the complexes with CD. Further analysis of these results implies calculation of the radiative and nonradiative deactivation rate constants, that can be obtained from the values of τ and Φ. The values of these rate constants are listed in Table 2. It can be seen that the radiative deactivation constant is about the same in the free pyronines and in the complexes. That is a reasonable result since this rate constant is usually not sensible to solvent effects. On the contrary, the nonradiative deactivation constant varies strongly upon complexation. The value for PY:CD is two times that of the free PY and a three-fold increase is observed for complex PB:CD with respect to free PB. This means that complexation favors the nonradiative deactivation pathways of these pyronines.
The increase of the nonradiative deactivation rate constant of pyronines and the parent molecules rhodamines in certain solvents has been extensively studied and several models have been proposed to explain it [8-11], where charge transfer from the amino groups to the xanthene moiety and hydrogen-bond interactions with the solvent molecules play important roles. In our case, the increase of the nonradiative deactivation rate constants in the complexes could be explained by the formation of an excited charge-transfer state that is strongly stabilized by interaction of the pyronines with the CD. Since the positive charge would be located at the xanthene ring in this charge-transfer state, stabilization would be due to the interaction of this ring with the electron-rich glucosidic oxygens of the CD cavity. A possible structural model of the complexes based in this interpretation is shown in Figure 5.
Figure 5. Possible structure of the complexes P:CD.
References
[1] J. Szejtli, T. Osa, J. L. Atwood , Comprehensive Supramolecular Chemistry 3,
Pergamon:
[2] K. Fujiji, C. Iwanaga, M.Koizumi Bull. Chem. Soc. Jpn. 1962, 35, 185.
[3] Mohamed El Baraka, M. Deumie, P. Viallet, T. J. Lampidis J. Photochem. Photobiol., A 1991, 56, 295.
[4] L. P. Gianneschi, T. Kurucsev J. Chem. Soc., Faraday Trans. 2 1974, 70, 1334.
[5] L. P. Gianneschi, A. Cant, T. Kurucsev J. Chem. Soc., Faraday Trans. 2 1977, 73, 664.
[6] R. L. Schiller, S. F. Lincoln and J. H. Coates, J. Chem. Soc., Faraday Trans. 1 1987, 83, 3237.
[7] W. Al-Soufi, M. Novo and M. Mosquera, Appl. Spectrosc. 2001, 55, 630.
[8] F. López Arbeloa, T. López Arbeloa, M. J. Tapia Estévez, and I. López Arbeloa, J. Phys. Chem. 1991, 95, 2203.
[9] T. López Arbeloa, F. López Arbeloa, P. Hernández Bartolomé and I. López Arbeloa, Chem. Phys. 1992, 160, 123.
[10] Y. Onganer, E. L. Quitevis J. Phys. Chem. 1992, 96 , 7996.
[11] B. Acemioglu, M. Arik, Y. Onganer J. Lumin. 2002, 97, 153.
comun_ECSOC.doc V1