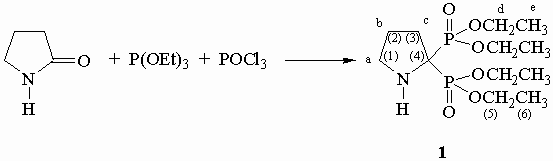
Tetraethyl(pyrrolidine-2,2-diyl)bisphosphonate
Gilles Olive 1* and Alain Jacques 2
1 Unité de physique et de chimie des hauts polymères,
Université catholique de Louvain, Bâtiment Bolztmann, Place
Croix du Sud, 1, B-1348 Louvain-la-Neuve, Belgium, Tel: +32 47 33 91, Fax:
+32 45 15 93, e-mail: [email protected]
2 Unité CSTR, Université catholique de Louvain,
Bâtiment Lavoisier, Place Louis Pasteur, 1, B-1348 Louvain-la-Neuve,
Belgium. E-mail: [email protected]
Received: 1 December 2001 / Accepted: 15 December 2001 / Published: 20 December 2001
Keywords: diphosphorylation, triethylphosphite, pyrrolidone,
bisphosphonate.
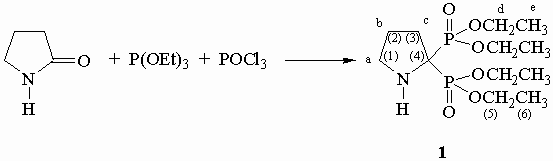
The synthesis of the title compound is already described [1, 2] and we report here the fully optimized procedure. In a double walls flask, under nitrogen, phosphorus oxychloride (20 ml; 0.22 mol) is added for 1 h 00 time to a mixture at -7.5 °C of 2-pyrrolidinone (9.3 g; 0.11 mol) and triethylphosphite (35.1 g; 0.21 mol; 1.9 eq.). The reaction mixture is stirred for 1 hour at room temperature and then poured over a mixture of ice (200 g) and ammonia 30 % (400 ml). The aqueous layer is extracted with methylene chloride (3x100 ml) and then the latter is removed to obtain a yellow oil. The oil is dissolved in 100 ml of methylene chloride. An aqueous solution of hydrochloric acid (10 ml of 32 % HCl solution, 190 ml of water) is added (check that pH 1) and the aqueous layer is washed with methylene chloride (3x100 ml). A solution of sodium hydroxide (20 g of NaOH in 200 ml of water) is added up to pH 10 and the aqueous layer is extracted with methylene chloride (4x100 ml). The organic layer is dried over sodium sulfate, filtered and the removal of the solvent affords 1 (20.9 g; 58 %) as a colourless oil.
Formula: C12H27NO6P2.
Molecular weight: 343.13 g.mol-1.
M.p: -36 °C.
B.p: 140 °C under 6.10-2 mmHg.
Rf: 0.39 (Acetone) ; 0.43 (CH2Cl2 / EtOH 19:1).
UV (EtOH, 25 °C): lmax (e) 205 (1622), 221 (1737), 264 (212) nm (mol-1.dm3.cm-1).
pKa: 3.5 - Krebs at 20 °C: 3.59 - Krebs at 37 °C: 3.47. [3]
IR (Neat): 3481 (nNH); 2982 (nCH2 ring + nCH3 As.); 2932 (nCH2 ethoxy); 2909; 2869 (nCH3 Sym.); 1670 (dNH); 1595; 1478; 1457 (dCH2 ring + ethoxy); 1392 or 1368 (dCHsp3 Sym.); 1324; 1293; 1243 (P=O); 1164 (P-OEt); 1098 (nC-N); 1044; 968; 794; 732; 645; 580; 536 cm-1.
Raman: 2979 (nCH2 ring + nCH3 As.); 2934 (nCH2 ethoxy); 2874 (nCH3 Sym.); 2778; 2725; 1594 (dNH in plane); 1482; 1458 and 1449 (dCH2 ring + ethoxy); 1394 or 1369 (dCHsp3 Sym.); 1289; 1240 (P=O); 1190; 1162 (P-OEt); 1100 (nC-N); 1027; 918; 877; 812; 757; 653; 291; 271 cm-1.
1H-NMR: d in ppm
referring to TMS (multiplicity, J in Hz) ; a, b, c, d and e as assigned
in scheme.
|
|
(MHz) |
|
|
|
|
|
|
|
|
|
(quint., 6.4) |
|
|
1.20 (t, 6.8) |
|
|
|
|
|
|
|
1.11 (t, 7.1) |
|
|
|
|
(quint., 6.4) |
|
|
|
|
55 °C = 328 K |
|
|
(quint., 7.2) |
|
|
|
|
|
|
|
(quint., 6.8) |
|
|
|
|
|
|
|
|
|
|
|
a JH-H
b JP-H
c Additional: 3.28 (ls, NH)
d Value of T1
13C-NMR: d in ppm
referring to TMS (multiplicity, JCP in Hz) (multiplicity, 1JCH
in Hz for CDCl3 and Acetone-d6) ; (1), (2), (3),
(4), (5) and (6) as assigned in scheme.
|
|
(MHz) |
|
|
|
|
|
|
|
|
|
(t, 139.4)a |
(t, 133.8)a |
(t, 135.4)a |
|
(tsext.c, 148.3)a 63.3 (t, 3.6) (tsext.c, 149.3)a |
(qh, 127.1)a 15.5b (qh, 127.1)a |
|
|
|
|
|
|
|
63.4 (t, 5.8) |
16.6 (t, 5.5) |
|
|
|
|
|
|
|
(q, 3.8) |
|
|
30 °C = 303 K |
|
|
|
|
|
62.6b |
|
|
55 °C = 328 K |
|
|
|
|
|
62.3 (t, 3.4) |
16.0 (t, 2.3) |
|
70 °C = 343 K |
|
|
|
|
|
62.5 (t, 3.5) |
|
|
|
|
(t, 138.9)a |
(t, 133.2)a |
(t, 133.8)a |
|
(t, 148.04)a 63.6 (d, 3.4) (t, 147.9)a |
(q, 130.5)a |
a Proton coupled (multiplicity, 1JCH
in Hz)
b Unresolved triplet
c Triplet of sextuplet
Variable temperature in DMSO at 63 MHz [4]
|
°C |
K |
|
|
|
|
|
|
|
|
|
|
|
|
|
62.6 (t, 3.6) |
|
|
|
|
|
|
|
|
62.5 (t, 3.6) |
|
|
|
|
|
|
|
|
62.5 (t, 3.6) |
|
|
|
|
|
|
|
|
62.5 (t, 3.5) |
|
|
|
|
|
|
|
62.5 (t, 3.7) |
|
|
|
|
|
|
|
|
62.5 (t, 3.7) |
|
a Only the central line
31P-NMR: d in ppm
referring to external 85 % H3PO4
d (CDCl3, 40 MHz): 22.5 ppm.
d (CDCl3, 162 MHz): 24.7 ppm.
d (C6D6, 40 MHz):
22.8 ppm.
da (Krebs at 20 °C, 162 MHz): 16.51
ppm - db (Krebs at 20 °C, 162 MHz): 24.09
ppm. [3]
da (Krebs at 37 °C, 162 MHz): 16.53
ppm - db (Krebs at 37 °C, 162 MHz): 24.18
ppm. [3]
T1 (CDCl3, 202 MHz): 1.89 s.
31P MAS NMR: d (10 KHz spinning at 200 K): 22.39 ppm. [5]
15N-NMR: d in ppm
referring to external CD3NO2: d
(CDCl3, 50 MHz): -341.6 (t, JNP = 8.6) ppm.
d (C6D6, 50 MHz):
-340.7 (t, JNP = 8.7) ppm.
17O-NMR: d in ppm
referring to external H2O: d (DMSO,
68 MHz): 95.4 (P=O) ppm.
d (Acetone d6, 68 MHz): 84.6
(P=O) and 53.9 (P-O) ppm.
References and Notes
1. Olive, G.; Le Moigne, F.; Mercier, A.; Rockenbauer, A.; Tordo, P.
J. Org. Chem. 1998, 63, 9095-9099.
2. Olive, G.; van Genderen, M. H. P. Magn. Reson. Chem. 2000,
38(5), 379-381.
3. Pietri, S.; Miolan, M .; Martel, S.; Le Moigne, F.; Blaive, B.;
Culcasi, M. J. Biol. Chem. 2000, 275(26), 19505-19512.
4. Olive, G.; Jacques, A. To be published.
5. Millot, Y. ; Olive, G.; Magusin, P. To be published.
Sample Availability: Available from the author and from MDPI.
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/