| Molbank 2003, M318 |
(±)-1-(4-Hydroxy-3-methoxyphenyl)-4-methyl-3-pentanol
Guy L. Plourde
University of Northern British Columbia, Department of Chemistry, 3333 University Way, Prince George, British Columbia, Canada, V2N 4Z9
Tel: 250-960-6694, Fax: 250-960-5545, E-mail: [email protected]
Received: 7 July 2002 / Accepted: 15 October 2002 / Published: 6 April 2003
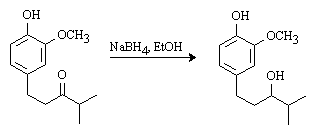
The discussion and purpose for the synthesis of this compound has been reported elsewhere [1]. To a cold
(0°C) solution of 1-(4-hydroxy-3-methoxyphenyl)-4-methyl-3-pentanone (190 mg, 0.86 mmol) in EtOH (15 mL) was added sodium borohydride (40 mg, 1.1 mmol, 1.3
eq). The solution was stirred at 0°C for 30 min., then at room temperature for
1 h. 10% HCl (10 mL) was added and the solution was stirred at room temperature for
2 h. The solution was concentrated in vacuo and the aqueous residue was extracted with dichloromethane (3 x 10 mL). The organic fractions were combined, dried
(MgSO4) and the solvent was evaporated in vacuo. Chromatography on silica gel (40%
EtOAc / hexanes) afforded a white solid (172 mg, 90%).
mp: 55-56°C.
IR (KBr) cm-1: 3397 (OH).
1H-NMR (CDCl3) d: 0.92 (d, 6H, J=7.8 Hz, CH3), 1.69 (m, 3H, H-2 and H-4), 2.59 (m, 1H, H-1a), 2.78 (m, 1H, H-1b), 3.40 (broad m, 1H, H-3), 3.88 (s, 3H, OCH3), 5.47 (s, 1H, exchangeable with D2O, OH), 6.72 (m, 2H, ArH-2 and ArH-6), 6.84 (d, 1H, J=8.7 Hz, ArH-5).
13C-NMR (CDCl3) d: 17.4 (C-5a), 19.0 (C-5b), 32.4 (C-1), 33.9 (C-4), 36.4 (C-2), 56.1 (OCH3), 76.4 (C-3), 111.2 (ArC-2), 114.5 (ArC-5), 121.1 (ArC-6), 134.5 (ArC-1), 143.8 (ArC-4), 146.6 (ArC-3).
MS m/e (rel %): 224 [M+] (83), 163 (44), 138 (62), 137 (100), 134 (44).
Anal. calc. for C13H20O3: C 69.60, H 8.99; found: C 69.55, H 8.78.
Acknowlegment
The author is thankful for the financial support provided by the University of Northern British Columbia.
Reference
1. Plourde G.L. Tetrahedron Letters 2002, 43, 3597-3599.
© 2003 MDPI. All rights reserved.