| Molbank 2004, M371 |
กก
Synthesis of 2-hydroxy-3-methoxy-5-(4-methoxyphenylazo) benzaldehyde. A new aldehyde for the preparation of biologically active molecules.
A. A. jarrahpour*, A. R. Esmaeilbeig and M. Zarei
Department of Chemistry, College of Sciences, Shiraz University, Shiraz 71454, Iran Tel. 0098 711 2284822, Fax: 0098 711 2280926, E-mail: [email protected], [email protected]
กก
Received: 8 October 2003 / Accepted: 20 February 2004 / Published: 24 February 2004
กก
Kewords : azoaldehyde ,p-anisidine , o-vanillin , Schiff bases, biological activity.
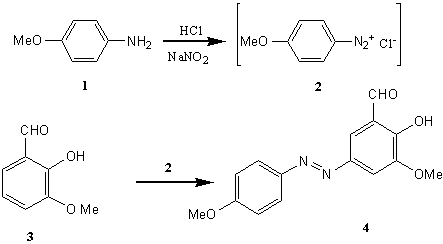
Diazo compounds have interesting biological activities [1-4]. It was shown that the presence of an azo functionality in many compounds is essential for the exhibition of pesticidal activity [5]. In addition, the existence of the methoxy groups on some molecules enhanced the biological activities [6]. As such, we synthesized a new aldehyde 4 possessing an azo as well as the methoxy groups. Reaction of this novel azoaldehyde with various aromatic amines afforded the corresponding Schiff bases. Their antibacterial and antifungal activities are under study to be presented in the near future.

Experimental: A solution of aqueous HCl (1.61 mL, 17.92 mmol ) in 20 mL water was added to the p-anisidine 1 (1.00 g, 8.12 mmol). It was stirred and cooled to 0 oC and then an aqueous solution of NaNO2 (0.62 g ,9.00 mmol ,8 mL H2O) was added dropwise. The so-formed diazonium chloride 2 was consecutively coupled with o-vanillin 3 (1.24 g , 8.12 mmol ), dissolved in 10.00 mL of aqueous 2N NaOH (0.80 g, 20.00 mmol ). The reaction mixture was stirred for 1 hour at 0 oC, and then allowed to warm-up slowly to room temperature. The brown precipitate thus obtained was filtered and washed with H2O (3 x 20 mL ). Then it was dissolved in CH2Cl2 and dried (Na2SO4), filtered, and the solvent was evaporated under reduced pressure [7]. The crude product was purified by column chromatography (silica gel, CH2Cl2/n-hexane, 1:1, V/V as eluant) to give 2-hydroxy-3-methoxy-5-(4-methoxyphenylazo)benzaldehyde 4 (1.77 g, 6.18 mmol) in 76.20 % yield.
m.p. 169-171 oC.
IR (KBr) (cm-1): 1600 (C=C) , 1670 (C=O) , 3030-3580 ( OH ) .
1H-NMR
(CDCl3) (250 MHz)![]() (ppm): 4.01(6H, s, 2OCH3), 7.07-8.26 (6H, m,
aromatic hydrogens), 10.06 (1H , s, CHO), 12.78 (1H, br, OH).
(ppm): 4.01(6H, s, 2OCH3), 7.07-8.26 (6H, m,
aromatic hydrogens), 10.06 (1H , s, CHO), 12.78 (1H, br, OH).
13C-NMR
(CDCl3) (62.90 MHz) ![]() (ppm): 56.82 (OCH3), 108.70-125.19 (aromatic
carbons), 196.91 (CHO).
(ppm): 56.82 (OCH3), 108.70-125.19 (aromatic
carbons), 196.91 (CHO).
MS (m/z,
%): 286 (M+, 65.5), 151 (![]() , 19.8), 135 (MeOC6 H4N2, 40.3),107
(MeOC6 H4, 100), 92 (C6 H4O, 20.3).
, 19.8), 135 (MeOC6 H4N2, 40.3),107
(MeOC6 H4, 100), 92 (C6 H4O, 20.3).
The authors thank the Shiraz University Research Council for financial support (Grant No. 81-SC-1495-C191 ).
1. P. Pathak , V. S. Jolly , K. P. Sharma Orient. J. Chem. 2000 , 16 (1) , 161-162
2. P. Pathak , V. S. Jolly , K. P. Sharma Orient. J .Chem. 2000 , 16 (3) , 493-494
3. Pradhan Alka , Pradhan Vinod ,V.S. Jolly Orient. J. Chem. 1994 ,10 ,73-74
4. V. S. Jolly , M. Pathak , R.jain J .Indian. Chem .Soc 1993 , 70 , 505-507
5. S. Samadhiya , A. Halve Orient. J. Chem. 2001, 17(1) , 119-122
6. A. Halve , A. Goyal Orient. J. Chem. 1996, 12 (1) , 87-88
7.B. S. Furniss ,A.J.Hannaferd ,V.Rogers, P.W.G.Smith, A.R.Tatchell Vogel ,s Textbook of Practical Organic Chemistry , 4th ed., Longman Inc. New York 1981 , p 716
กก
Sample Availability : Available from MDPI.
© 2004 MDPI. All rights reserved