| Molbank 2004, M379 |
Lajos Kov¨˘cs1, *, P¨¦ter Forg¨®2 and Zolt¨˘n Kele1
1Department of Medicinal
Chemistry,
2Department of Organic Chemistry, University of Szeged,
D¨®m t¨¦r 8, H-6720 Szeged, Hungary. Fax: +36-62-54 59 71
*E-mail: [email protected]
Received: 16 October 2003 / Accepted: 11 March 2004 / Published: 29 March 2004
ˇˇ

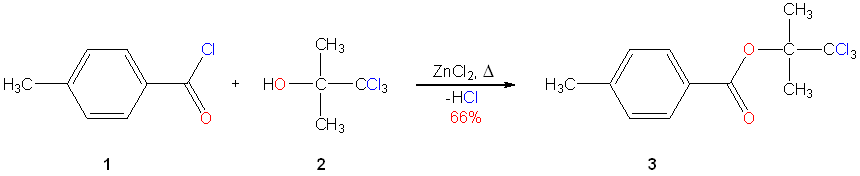
In our previous work we have demonstrated the utility of the 2,2,2-trichloro-1,1-dimethylethyl (b,b,b-trichloro-tert-butyl, Tcb) group for the protection of carboxylic acids [1]. Herein we would like to describe the preparation of one of their representatives, the title aromatic ester. This compound was mentioned by Gupta and Srivastava [2] but it was not described in detail.
Experimental: Freshly prepared anhydrous zinc chloride (1.90 g, 13.9 mmol) was chilled in an ice-bath, then 4-methylbenzoyl chloride (10.75 g, 69.5 mmol) and anhydrous b,b,b-trichloro-tert-butanol (8.225 g, 46.3 mmol, prepared by in vacuo drying of the commercial hemihydrate over phosphorus pentoxide) were added, each in one portion. The flask was removed from the ice-bath and gently shaken to initiate the evolution of hydrogen chloride. When the gas evolution had subsided the reaction mixture was heated at 100 ˇăC for 2 h. The mixture was diluted with diethyl ether (200 mL), extracted with cold satd. NaHCO3 solution (3x100 mL) and brine (100 mL), respectively. The organic phase was dried (MgSO4) and evaporated. The oily residue solidified upon standing (13.167 g, 96%, TLC: slightly contaminated) and it was recrystallized from ethanol (15 mL) to afford the product (9.088 g, 66%).
Mp. 84.6-86.3 ˇăC (lit. [2] 85 ˇăC).
TLC: light petroleum-ethyl acetate 95:5, Rf: 0.55.
Anal. calcd. for C12H13Cl3O2 (295.589): C, 48.76; H, 4.43; Cl, 35.98%; found C, 48.90; H, 4.37; Cl, 35.72%.
1H NMR (CDCl3, 500 MHz, ppm): 2.04 (s, 6H, (CH3)2C), 2.42 (s, 3H, arom. CH3), 7.25 (d, 2H, J=8.3 Hz, arom. CHs), 7.95 (d, 2H, J=8.3 Hz, arom. CHs).
13C NMR (CDCl3, 125 MHz, ppm, assignment based on HMBC and HMQC experiments, asterisks denote interchangeable assignments): 21.41 ((CH3)2C*), 21.66 (arom. CH3*), 88.98 (CCl3), 106.54 ((CH3)2C), 127.94 (C-4), 129.16 (arom. CHs), 129.85 (arom. CHs), 143.94 (C-1), 164.52 (COO).
CI-MS (isobutane, m/z): 297, 295 ([M+H]+, isotopic peaks), 263, 261 ([M-Cl]+, isotopic peaks). The calculated peak distributions correspond to the expected structure.
References:
1. L. Kov¨˘cs, P. Forg¨®, Z. Kele: Fourth International Electronic Conference on Synthetic Organic Chemistry, abstr. no. a0076 (http://www.unibas.ch/mdpi/ecsoc-4/a0076/a0076.htm).
2. I. Gupta, N. P. Srivastava: Recl. Trav. Chim. Pays-Bas, 1956, 75, 48-50.
Sample availability: sample available from the authors.
© 2004 MDPI. All rights reserved.