| Molbank 2004, M390 |
﹛
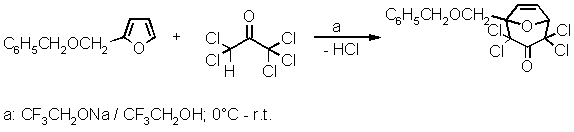
1-Benzyloxymethyl-2,2,4,4-tetrachloro-8-oxabicyclo[3.2.1]oct-6-en-3-one
G邦nter Kreiselmeier and Baldur Föhlisch*
Institut
f邦r Organische Chemie der Universität Stuttgart, Pfaffenwaldring 55, D-70569
Stuttgart, Germany
Fax: (+ 49) 711/6854269;
E-mail: [email protected]
Received: 8 March 2004 / Accepted: 17 March 2004 / Published: 29 March 2004

A mixture of 2-benzyloxymethylfuran[1] (1.88 g, 10 mmol) and pentachloroacetone[2] (2.30 g, 10 mmol) was cooled in an ice bath. With magnetic stirring, a 1-molar solution of sodium 2,2,2-trifluoroethoxide in 2,2,2-trifluoroethanol[3] (14 mL, 14 mmol) was added dropwise, within 2.5 hours. Sodium chloride precipitated. The ice bath was removed, and stirring was continued for 3 h at room temperature. Then, 25 mL of water was added dropwise; the sodium chloride dissolved, and crystals of the title compound were forming. To complete the crystallization, the mixture was stirred at 0∼ C for 1 hour. The solid was filtered and washed with water to the point of neutrality[4]. After a final wash with a few mL of ice-cold 50% aqueous ethanol the colourless crystals were dried over P4O10 to yield 2.52 g (66%) with m.p. 99每99.5 ∼C.
TLC (silica, petroleum ether/ethyl acetate, 10:1 v/v): A greenish-brown spot emerged after spraying the sheet with vanillin/sulfuric acid reagent followed by heating with a hot-air gun; Rf = 0.42.
1H-NMR (250 MHz, CDCl3): d= 4.19 (center of an AB sub-spectrum with dA = 4.27, dB = 4.11, JAB = 11.5 Hz, diastereotopic BnOCH2), 4.65 (center of an AB sub-spectrum with dA = 4.70, dB = 4.59, JAB = 12.1 Hz, diastereotopic PhCH2), 5.26 (appearing as a &d*, line distance 1.6 Hz, 1H, X part of an ABX sub-spectrum that could not be fully analysed, H-5), 6.48每6.54 (6 lines of the AB part of the ABX sub-spectrum, JAB = 6.0 Hz, 2 H, H-6 and H-7), 7.24每7.40 (m, 5 H, C6H5).
13C-NMR/DEPT (62.9 MHz, CDCl3): d= 67.6 (+, BnOCH2), 74.05 (+, PhCH2), 82.2 (Cq, C-4 or C-2), 85.1(Cq, C-2 or C-4], 87.6 (-, C-5), 94.3 (Cq, C-1), 127.7 (-), 128.0 (-), 128.5 (-), [C-2每C-6 of the phenyl group], 133.2 (-, C-6 or C-7), 136.0 (-, C-7 or C-6), 137.25 (Cq, C-1 of the phenyl group), 184.6 (Cq, C-3).
IR (KBr): 3090, 3075, 3040, 3010 (=C-H), 2960, 2935, 2910 (sh), 2900, 2855 (C-H), 1760, 1740 (C=O), 1595 cm-1 (C=C).
Anal. Calcd. for C15H12Cl4O3 (382.1): C, 47.16; H, 3.17; Cl, 37.12. Found: C, 47.35; H, 3.30; Cl, 36.98.
References and Notes
1. Achmatowicz, O.; Burzynska, M. H. Tetrahedron 1982, 38, 3507每3513.
2. Bugrova, L. V.; Rudnev, G. K.; Kristich, A. I.; Radchenko, V. I.; Mishchenko, L. F. Zh. Prikl. Khim. (Leningrad) 1973, 46, 1529每1533; J. Appl. Chem. USSR 1973, 2, 1627每1631.
3. a) Föhlisch, B.; Gehrlach, E.; Herter, R. Angew. Chem. 1982, 94, 144; Angew. Chem. Suppl. 1982, 94, 241; Angew. Chem., Int. Ed. Engl. 1982, 21, 137. 每 b) Sendelbach, S.; Schwetzler-Raschke, R.; Radl, A.; Kaiser, R.; Henle, G. H.; Korfant, H.; Reiner, S.; Föhlisch, B. J. Org. Chem. 1999, 64, 3398每3408.
4. This work-up, i.e. precipitation by careful addition of water, was also used for the preparation of oxabicyclic compounds from pentachloroacetone and furan (71% yield, m.p. 90每91 ∼C), 2-methylfuran (63%, m.p. 56每57 ∼C), 3-methylfuran (88%, m.p. 89每90 ∼C), 2,5-dimethylfuran (89%, 112每113 ∼C), and 2-(3-butenyl)-3-methylfuran (86%, m.p. 59每60 ∼C)[3]. If the products do not crystallize immediately, the oily precipitate becomes solid on scratching with a glass rod. The compounds are pure enough for dechlorination, but may be purified by sublimation in vacuo.
﹛
© 2004 MDPI. All rights reserved.
﹛
﹛
﹛