[B0001]
Colour Encoded Parallel Synthesis
SINTEF Applied
Chemistry, P.O. Box 124 Blindern, N-0314 Oslo, Norway.
E-mail: [email protected]
Received: 9 August 1999 / Uploaded: 10 August 1999
Abstract
A method based on colour coding is presented which allows for the facile deconvolution of multi-component reactions in library synthesis.
Introduction
Multi-component reactions are very important in library synthesis.1 They allow for the rapid incorporation of diversity elements onto a backbone or template in one step, often in high yield under mild conditions. However, multi-component reactions have been described as being "un-encodable" making the deconvolution of libraries based on these reactions difficult.2 Herein is described a straight forward method that can be used for deconvolution purposes. Two reactions, the Passerini and Ugi condensations, were examined as examples of encodable multi-component reactions.

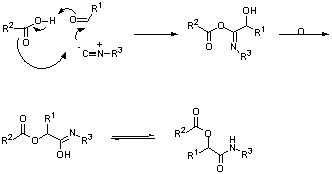
Fig 1: Passerini reaction
The Passerini reaction is a 3-component condensation involving the reaction of an acid with a carbonyl compound and an isocyanide (Fig 1). Similarly the Ugi reaction is a 4-component condensation involving the reaction of a carbonyl compound with an amine in the presence of both an acid and an isocyanide (Fig 2).
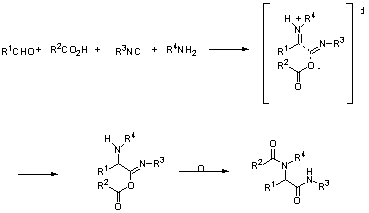
Fig 2: Ugi reaction
Results and Discussion
Nine building blocks were considered for the construction of a 36-membered library via 3- and 4-component condensations. These composed of three carboxylic acids, two aldehydes, two isocyanides and two amines (Fig 3). 12 a-(Acyloxy)carboxamides could be realised from Passerini condensation of the acid, aldehyde and isocyanide components (3x2x2) and 24 a -aminoacylamides from Ugi condensation of these building blocks with the amine components (3x2x2x2).
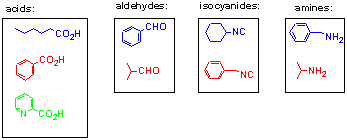
Fig 3: Building blocks used in multi-component reactions
In order to facilitate deconvolution of the library each building block was assigned a colour unique within its class, i.e. only one acid, one aldehyde, one isocyanide and one amine were assigned the colour blue, etc. (Fig 3). In addition each reaction vessel was colour bar-coded according to its constituent starting materials. For the Passerini reactions the first position on the bar code was defined as the acid component, the second as the aldehyde and the third as the isocyanide. In the case of the Ugi reactions, the bar code was positionally defined as first acid, second amine, third aldehyde and fourth isocyanide (Fig 4). These characteristic coloured bar codes were then used as a means of identifying the expected products via their synthons and hence starting materials.
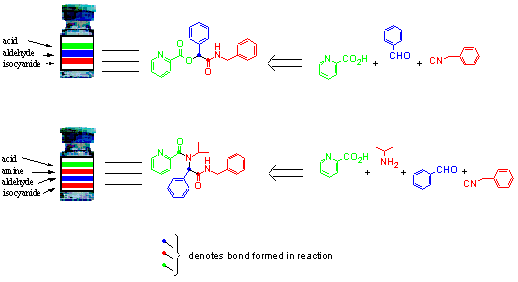
Fig 4: The coloured bar code yields information as to the composition and origin of final products
The 36 compound library was synthesised in solution by multi-component condensations of the reactants in Fig 3. All compounds were isolated as single racemic mixtures, since a stereocentre is formed during reaction from the carbonyl source. The 36-membered library composed of 12 products from the Passerini (Fig 5) and 24 products from the Ugi reaction (Fig 6). All reaction products were analysed by TLC and HPLC and a select few by 1H NMR and GC/MS. The coloured bar codes allowed for the rapid deconvolution and thereby identification of all library members. Although the final products were not tested for biological activity, the colour encoding of a library should also assist in the visual determination and mapping of structure activity relationships. This can be achieved simply by the analysis of data obtained from screening together with the composition of the coloured units in the final products.
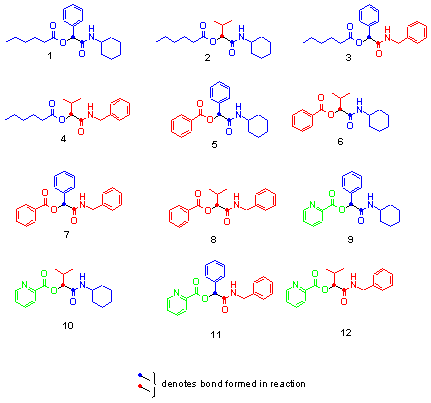
Fig 5: a-(Acyloxy)carboxamides produced by Passerini reaction
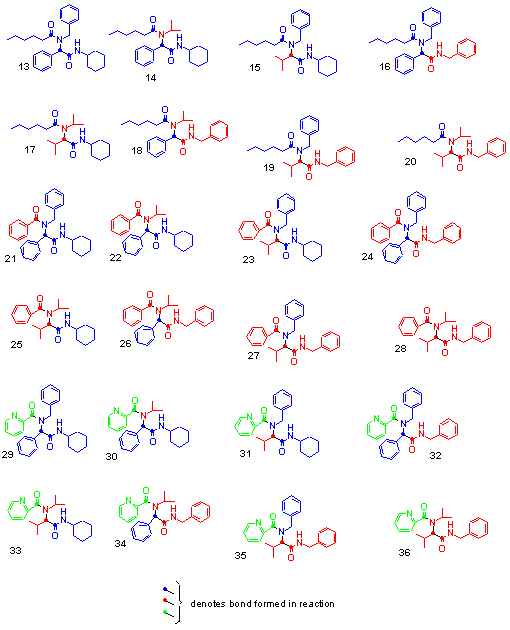
Fig 6: a-Aminoacylamides produced by Ugi reaction
Conclusion
A library of 36 compounds was encoded by the use of just three colours. The colour encoding of building blocks in multi-component reactions allows for the rapid deconvolution of reaction products. This simple colour bar-coding method should allow for the encoding of significantly larger libraries, e.g. it is possible to encode 10 000 reaction products from a 4-component reaction by the use of just 10 different colours on a four-coloured bar code (10x10x10x10).
Experimental
Passerini reaction: 1 M Solutions of the reactants in dichloromethane were prepared. The isocyanide solutions (100 mL) were added by pipette to the aldehyde solutions (300 mL), followed by the carboxylic acid solutions (100 mL). The reaction vessels were then shaken vigorously on a Capmix for 10 s before being agitated on a Coulter Mixer for 60 h. Reaction products were then purified and isolated.3 Sample 1H NMR data for 7 (major rotamer) (300 MHz, CDCl3) d 8.08 (2H, d, J = 6.5 Hz), 7.64-7.18 (13H, m), 6.60 (1H, bs), 6.39 (1H, s), 4.48 (2H, t, J = 8.3 Hz).
Ugi reaction: 1 M Solutions of the reactants in methanol were prepared. The reagents were added to each other in order of their reaction, ie aldehyde (125 mL), amine (125 mL), isocyanide (100 mL) and carboxylic acid (100 mL). The reaction vessels were then shaken vigorously on a Capmix for 10 s before being agitated on a Coulter Mixer for 60 h. Reaction products were then purified and isolated.3 Sample 1H NMR data for 21 (300 MHz, CDCl3) d 7.49-7.04 (15H, m), 5.63 (1H, bs), 5.48 (1H, s), 4.70 (1H, d, J = 14.0 Hz), 4.50 (1H, d, J = 14.0 Hz), 3.85 (1H, m), 1.93-1.79 (2H, m), 1.70-1.50 (4H, m), 1.45-1.22 (2H, m), 1.18-0.90 (2H, m).
Biographical Summary
Lorenzo received a BSc (Hons) in Applied Chemistry (1988) followed by a doctorate in organic chemistry (1992) at the University of Salford, UK. Lorenzo undertook his PhD under the direction of Dr Barry Lygo in the field of natural product synthesis. During his time at Salford, Lorenzo also had periods working at Unilever Research, ICI Pharmaceuticals and Glaxo Greenford Research. Lorenzo enjoyed a postdoctoral position in Norway working with Professor Kjell Undheim in the field of radical chemistry. Thereafter he undertook a further two years as a postdoctoral fellow at the University of Pennsylvania, USA with Professor Madeleine Joullie´ in the area of natural product chemistry. Projects under his current direction are in the areas of molecular diversity/ combinatorial chemistry and liquid crystals. Lorenzo is a Chartered Chemist and has been a Member of the Royal Society of Chemistry since 1996. He is also a member of the American and Norwegian Chemical Societies.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with b0001 as the message subject of your e-mail.