Development of a potentially biomimetic methodology
General remarks and comparison of the Shemin mechanism with the Knorr pyrrole synthesis
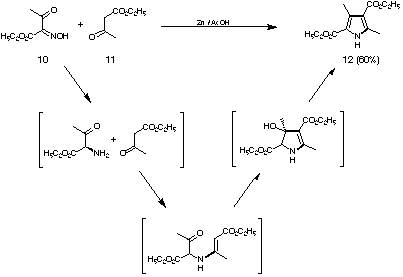
Novel pyrrole synthesis based on Shemin's proposal for the biosynthesis
General remarks and comparison of the Shemin mechanism with the Knorr pyrrole synthesis Concentrating only on the step connecting the two substrate molecules via a covalent bond for the first time, there have been three mechanisms postulated so far for the biosynthesis of porphobilinogen (4).[24-26] In two of these mechanisms the central decisive step for the building of the pyrrole ring is the formation of the carbon-carbon bond between C3 of one 5-aminolevulinic acid (5) reacting as a nucleophile and the keto function of the other 5-aminolevulinic acid. It was assumed that the nucleophilic 5-aminolevulinic acid partner is bound to the enzyme as an enamine. One would expect that the formation of the central carbon-carbon bond should be slowest and therefore the rate determining step of the biosynthetic sequence. This sequence is in remarkable contrast to the mechanism of the Knorr pyrrole synthesis (see Figure 7).[27]

Figure 7: Mechanism for the Knorr pyrrole synthesis.
For the Knorr pyrrole synthesis the steps leading to the pyrrole nucleus
are the same, but the sequence is clearly different from the Shemin mechanism for the
biosynthesis (see Figure 8). In the Knorr synthesis the carbon-nitrogen bond is formed
first and the aldol-like carbon-carbon bond forming reaction is therefore an
intramolecular process.
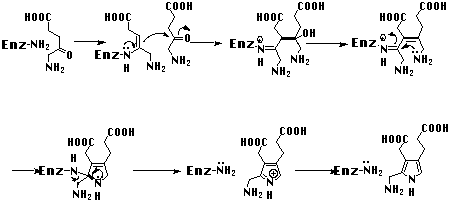
Figure 8: Mechanism proposed by Shemin for the biosynthesis of porphoblinogen (4).
Following the mechanistic reasoning first proposed by Shemin, one can ask the question if pyrroles can be synthesised using the same sequence of transformations and if it will be possible to synthesise even porphobilinogen (4) in a biomimetic way using such a methodology?
Based on this mechanistic question a novel synthesis of pyrroles could
be developed. The key step is the Mukaiyama crossed aldol reaction between
regioselectively generated silyl enol ethers 13 and 16 and azido ketals 14 (see Figure
9).[28] The Mukaiyama crossed aldol reaction forms the crucial carbon carbon bond.[29]
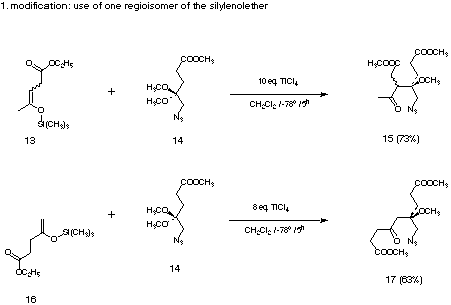
Figure 9: The crossed-aldol reaction using the pure silyl enol ethers 13 and 16.
In the second step the azido group is reduced either using triethylphosphine to induce the Staudinger reaction followed by an aza-Wittig reaction. The triethylphosphine gives the water soluble triethylphosphine oxide, which can be easily removed by extraction (see Figure 10).
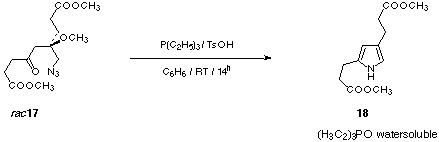
Figure 10: Modified Staudinger reaction of rac-17 forming the pyrrole 18.
Catalytic reduction is another mild method to transform the azido group
into the corresponding amine. Using palladium on charcoal as catalyst and methanol as
solvent the aldol product rac-15 could be reduced to 19 (see Figure 11). The amino
ketone formed spontaneously the corresponding pyrrole. The work-up using these conditions
was very convenient.
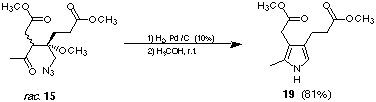
Figure 11: Catalytic reduction of 15 forming the pyrrole 19.
The new two-step pyrrol synthesis is especially effective for the
synthesis of mono-, di-, tri- and tetraalkylpyrroles in good yield (see Figure 12).[28]
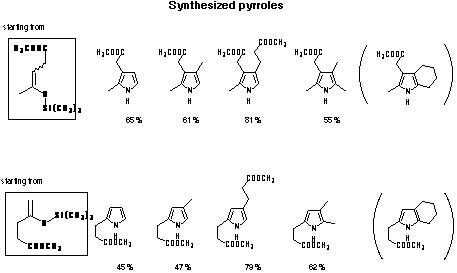
Figure 12: Pyrroles synthesised using the Mukaiyama crossed aldol condensation.
The synthesis is complementary to the classical Knorr pyrrole synthesis. It allows to
introduce the side chains at the correct positions and with the needed functionalities
already in the pyrrole forming step. The reaction conditions for the pyrrole formation are
sufficiently mild to allow also the isolation of highly sensitive pyrroles. At this stage
of the project we hoped to be able to apply our reaction conditions to a synthesis of
porphobilinogen (4) avoiding many of the pitfalls of the former synthesis.
![]()
![]() Chemical Synthesis of Porphobilinogen
Chemical Synthesis of Porphobilinogen
![]() "A Novel Synthesis of Porphobilinogen: Synthetic And Biosynthetic
Studies"
"A Novel Synthesis of Porphobilinogen: Synthetic And Biosynthetic
Studies"
Christiane Bobillier Neier / August 1999