Inhibition Studies of Porphobilinogen Synthase from Escherichia coli
Inhibition tests and inhibition type for porphobilinogen synthase In order to obtain kinetic information on the biosynthesis of porphobilinogen synthase we have undertaken a systematic search of the inhibition behaviour of this enzyme isolated from Rhodopseudomonas spheroides and from Escherichia coli.[38,53,54] The intention of these studies was to accumulate sufficient knowledge about the recognition site of this enzyme, so that this information will help to deduce conclusions about the mechanism. To be able to draw valid conclusions we had to analyse systematically the behaviour of analogues of the substrate and of analogues of the product. The result of these studies should allow us to interpret the findings obtained from studies of analogues of postulated intermediates. With a firm knowledge about the factors important for the recognition at the active site, it should be possible to interpret the whole body of information in a coherent way. Finally we hoped that the best of our inhibitors will contribute to the structural analysis of porphobilinogen synthase, as soon as the crystal structures become available and as soon as co-crystallization becomes feasible.
The majority of our studies were done with the enzyme isolated from an
overproducing strain from Escherichia coli gratefully put at our disposal by Dr.
Charles Roessner and Professor Ian A. Scott from Texas A&M (see Figure 19).[55]

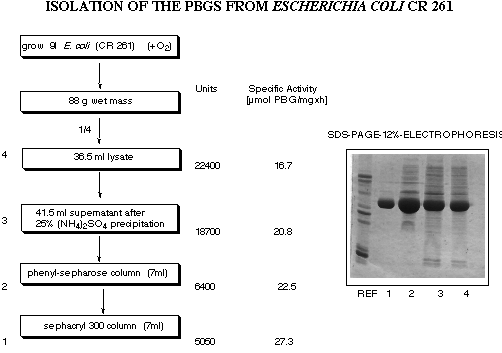
Figure 19: Isolation and purification of porphobilinogen synthase obtained from E. coli CR 261.
The test of the enzyme kinetics and activity is based on a modified Ehrlich test, which
allows to determine the amount of porphobilinogen (4) formed by measuring the absorption
at 554 nm of the chromophor obtained by treating the test solution with p-dimethylamino
benzaldehyde (see Figure 20).
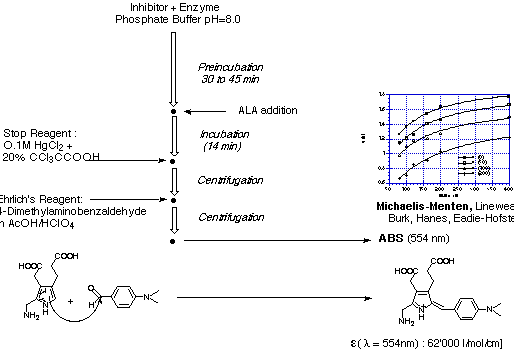
Figure 20: Ehrlich test for the determination of the kinetics of porphobilinogen
synthase.
The kinetic data obtained are highly reproducible and followed nicely Michaelis-Menten kinetics. This result came as a surprise to us, because an enzyme using two equivalents of the very same molecule as substrate should follow Michaelis-Menten kinetics only under very special conditions. Testing the kinetics of porphobilinogen synthase under a variety of concentration conditions we could show that the simple Michaelis-Menten kinetics is nicely followed if the concentration of the natural substrate 5-aminolevulinate (5) is 80 mM or bigger. If however the concentration of 5-aminolevulinate (5) is varied between 4mM and 80 mM significant deviation from the linear dependence predicted by the Michaelis-Menten kinetics can be observed (see Figure 21).
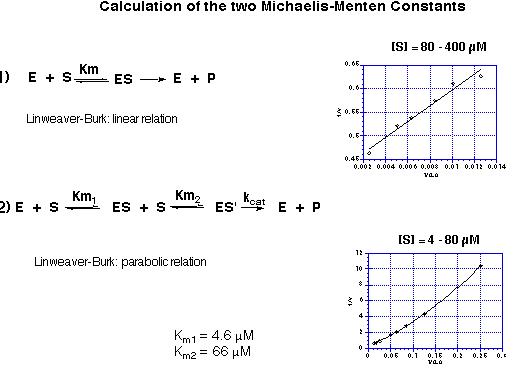
Figure 21: Dependence of the kinetic behaviour on the concentration range used for
the substrate: a) linear Michaelis-Menten behaviour for concentrations of
5-aminolevulinate (5) of 80 mM or bigger;
b) parabolic behaviour for concentrations of 5-aminolevulinate of 4 - 80 mM.
The linear part of the correlation allows to determine the Michaelis constant KM following the classic analysis of enzyme kinetics. The parabolic curve could be analysed using non-linear regression. The kinetic model used was a steady-state model based on the sequential formation of two Michaelis complexes with two different Michaelis constants. The two Michaelis constants KM1 and KM2 could be determined. The Michaelis constant KM1 for the tight binding substrate was 4.6 mM and the Michaelis constant KM2 for the loosely bound substrate was 66 mM which corresponds nicely to the value determined from the linear Michaelis-Menten correlation. This two values correlate perfectly well with the observation made for the single turn-over experiments which indicated that the recognition of the first substrate was sufficiently more tight, so that the sequence of recognition could be determined experimentally. The difference between the two KM values explains also the fact that a standard Michaelis-Menten kinetics can be observed using substrate concentrations which are in the range of the second Michaelis-Menten constant or higher.
For the inhibition experiments experimental conditions are used, which should give normal Michaelis-Menten kinetics (see Figure 22). Under these conditions the recognition of the second substrate at the A-site of the enzyme is determined. Such a behaviour is characterised by a clear unequivocal competitive inhibition. When the inhibition behaviour of a specific inhibitor becomes more complex, this is a clear sign that not only competition for the binding of the second substrate at the A-site plays a role, but that a double interaction can occur. If an inhibitor interacts with the A- and the P-site this double interaction leads to mixed or uncompetitive inhibition. If the interaction becomes thermodynamically stronger than the interaction with the natural substrate, then slow tight binding can be observed. Under these experimental conditions the type and the site(s) of interaction of a specific inhibitor can be deduced from the inhibition type determined with the help of the kinetic analysis. Studying more than 100 inhibitors this correlation could be verified and has become an important framework for the interpretation of the experimental results.
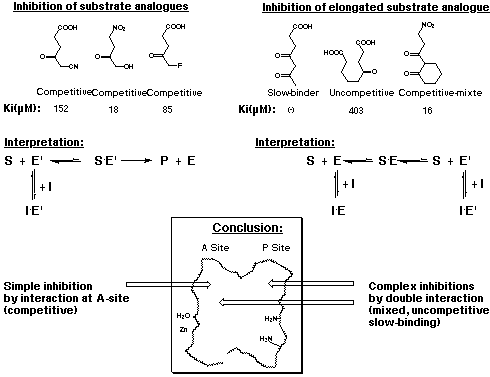
Figure 22: Inhibition type as a function of the sites of interaction
The most important application of this interpretation was the study of a series of diacids which were considered to be analogues of the postulated intermediates (see Figure 23).
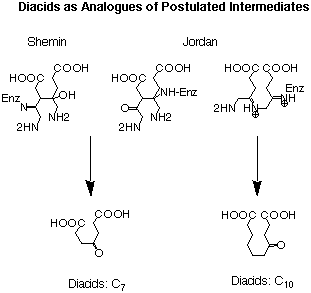
Figure 23: Comparison of the structures postulated as intermediates by Shemin and
Jordan with diacids used as inhibitors.
After the formation of the first bond uniting the two substrates a diacid is created for both postulated mechanisms. The difference between the two proposals is the fact that in the Shemin mechanism the diacid is a derivative of pimelic acid, whereas in the Jordan mechanism the diacid intermediate is a derivative of sebacic acid. In order to obtain good recognition a g-keto function is added, which allows the inhibitor to interact at three points with the active site.
The systematic study of the g-keto
dicarboxylic acids from C5 to C12 gave a clear picture (see Figure 24). The first three
dicarboxylic acids C5 to C7 are weak competitive inhibitors with inhibition constants KI
between 8'500 and 10'400 mM. Going to C8 and C9 the type of
inhibition changes and the value for the inhibition constant diminishes dramatically. The
inhibition constants KI are almost a factor of 100 smaller and uncompetitive
behaviour is observed. C10 is under our conditions an irreversible inhibitor, but it
should probably be better classified as slow tight binding inhibitor. Finally the
dicarboxylic acids C11 and C12 are slow binders.
Cx |
Compound |
Ki (ÁM) |
Inhibition type |
C5 |
|
8'500 |
Competitive |
C6 |
|
10'400 |
Competitive |
C7 |
|
8'600 |
Competitive |
C8 |
|
82 |
Uncompetitive |
C9 |
|
450 |
Uncompetitive |
C10 |
|
(-) |
Irreversible |
C11 |
|
(-) |
Slow-binder |
C12 |
|
(-) |
Slow-binder |
Figure 24: Results of the inhibition studies of the g-keto dicarboxylic acids C5 to C12.
The important conclusions from this series of inhibition studies is clear (see Figure 25). The g-keto dicarboxylic acids, which resembles the intermediates postulated by Jordan are tightly bound, so much so that they show an irreversible behaviour. The intermediate which imitates the intermediate postulated by Shemin however seems to be only recognised as analogue of the substrate without any additional site of interaction with the enzyme. As a consequence, only a weak interaction between the C7 dicarboxylic acid and the enzyme is observed, which is reflected by the high unspectacular value for KI. An additional argument is the observation that the two diastereroisomers rac-30 and rac-31 which imitate more closely the intermediate postulated by Shemin are even weaker inhibitors than the simple 4-oxo-pimelic acid. The values of KI are 11'900 and 17'000 mM respectively. The obvious interpretation of these results is that the 4-oxo sebacic acid is recognised at the two carboxylic acid ends of the molecule and the keto function forms a Schiff base with the active site lysine of the enzyme as additional point of recognition. This three point recognition leads to a quasi irreversible behaviour of this inhibitor. Inhibitors which are slightly too short or slightly too long still are strongly bound to the active site, but they show either slow-binding behaviour or good recognition, which means a small KI value and uncompetitive behaviour. Inhibitors, where the distance between the two carboxylic acid ends is too small, are "only" recognised as substrate analogues and show therefore competitive and not very efficient inhibition behaviour. Based on these observations clearly the Jordan mechanism is preferred.
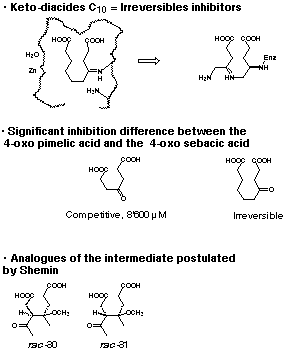
Figure 25: Interpretation of the inhibition results using the g-keto dicarboxylic acids.
![]()
![]() "A Novel Synthesis of Porphobilinogen: Synthetic And Biosynthetic
Studies"
"A Novel Synthesis of Porphobilinogen: Synthetic And Biosynthetic
Studies"
Christiane Bobillier Neier / August 1999