Introduction
Importance of the "pigments of life"
Monopyrroles as natural products
Biosynthesis of porphobilinogen
Previous synthesis of porphobilinogen
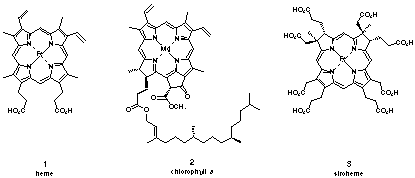
Importance of the "pigments of life" The studies of the tetrapyrrolic dyes, which have been called the "pigments of life"[1] have attracted the attention of chemists and biologists since their discovery. The importance of the "pigments of life" as crucial cofactors for processes like photosynthesis, oxygen transport, oxidation processes, methane synthesis and for a series of unusual rearrangements illustrates well the central role played by this class of natural products 1 - 3 (Figure 1).[2]

Figure 1: Some important "pigments of life"
Over a period of about 70 years at least six Nobel prices were awarded in Chemistry alone for research obtained working on problems related to the tetrapyrrolic natural products.(R. Willstätter, H. Fischer, D. Crowfoot-Hodgkin, M.F. Perutz , J.C. Kendrew, R.B. Woodward, J. Deissenhofer, R. Huber, H. Michl).
Relatively few mono-pyrrolic natural products have been reported in the literature. Most of these natural mono-pyrroles are stabilised by an electron-withdrawing substituent or by an aromatic ring. Without these substituents the electron rich pyrrole ring is easily polymerised or auto oxidised.
Porphobilinogen (4) a trialkylsubstitued pyrrole is a remarkable exception to this rule (Figure 2). The lack of stabilising substituents confers a high reactivity to porphobilinogen (4). The biosynthesis of the tetrapyrrolic "pigments of life" makes use of this high reactivity. About 1010 tons of chlorophyll and more than 4*105 tons of hem are synthesised each year [3,4].
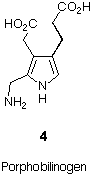
Figure 2: Porphobilinogen (4).
Porphobilinogen (4) is the second dedicated intermediate in the biosynthesis of tetrapyrroles.[5]
The tetrapyrrolic skeleton of all "pigments of life" is synthesised in a highly convergent way, starting with 8 molecules of d-aminolevulinic acid (5). 5-aminolevulinic acid (5) is then condensed to porphobilinogen (4), which itself tetramerises to form uroporphyrinogen III (6) (Figure 3).
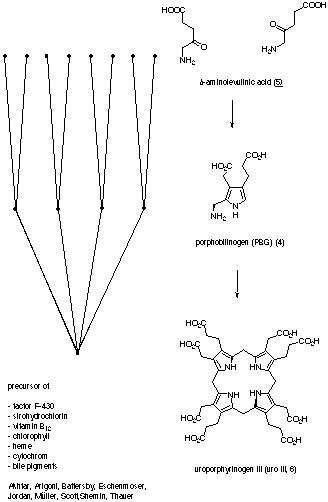
Figure 3: Biosynthesis of Uroporphyrinogen III (6)
The tetramerisation of porphobilinogen (4) could be achieved without the help of an
enzyme (Figure 4).[6] Porphobilinogen (4) forms uroporphyrinogens 6 - 9 induced by heat
and in the presence of mineral acid. The chemical reactivity of porphobilinogen (4) leads
to the formation of the next biosynthetic intermediate without the help of an enzyme. This
enzymatic transformation might be called an example of a chemomimetic biosynthesis.[7]
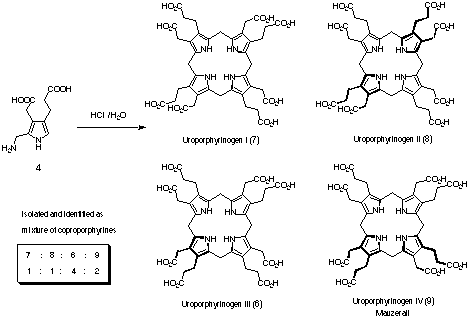
Figure 4: Tetramerisation of porphobilinogen (4).
These observations immediately raise the question of the mechanism for the
transformation catalysed by porphobilinogen synthase (=PBGS) and of the comparison between
the enzyme catalysed mechanism and its chemical analogue the Knorr pyrrole synthesis (see
Figure 5).
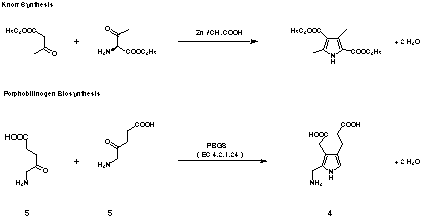
Figure 5: Comparison between Knorr pyrrole synthesis and porphobilinogen (4) biosynthesis.
For the dimerisation of 5-aminolevulinate(5) to porphobilinogen (4) a *G = -16.9 kcal/mol and for the tetramerisation of porphobilinogen (4) to uroporphyrinogen a *G = -34.6 kcal/mol were calculated for the gas phase reactions.[8] The biosynthesis of tetrapyrroles liberates free energy. This observations were taken as arguments in favour of a spontaneous formation of tetrapyrroles [9].
The importance of the "pigments of life" and the elegance of the biosynthetic pathway was a strong motivation to develop chemical synthesis of porphobilinogen (4). In recent years the need for good analytical methods to determine low levels of lead poisoning has renewed the interest in the synthesis of porphobilinogen (4).[10]
The synthesis of porphobilinogen (4) has attracted the attention of the chemists for different reasons: In the beginning the synthetic efforts were undertaken to prove the structure;[11] afterwards the interest was mainly focused on the synthesis of porphobilinogen (4) labelled at a specific positions in order to use it in the studies of the biosynthesis.[12,13] Finally the interest to develop synthesis of porphobilinogen (4) was renewed, when it became clear that the enzyme synthesising porphobilinogen (4), porphobilinogen synthase, is a very sensitive indicator for lead poisoning. Despite an exorbitant price which is almost three factors of 10 higher than the price for gold there have been only a limited number of fundamentally different approaches to porphobilinogen (4) or to analogues of porphobilinogen (4) been reported in the literature. Especially surprising is the fact, that since 1979 when the synthesis of porphobilinogen (4) has been reviewed by Frydman the last time,[14] only very few new results have been reported.[15]
Six synthetic strategies have been reported for the synthesis of porphobilinogen (4) (see Figure 6).
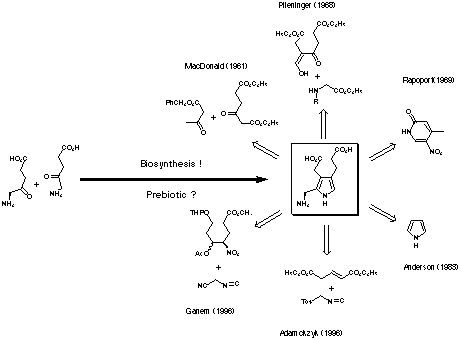
Figure 6: Synthetic strategies for the synthesis of porphobilinogen (4) compared with the biosynthesis.
The first and historically the oldest strategy uses a classic Knorr synthesis to obtain a suitable precursor.[16] To obtain the correct substitution pattern the group of MacDonald has invested a considerable amount of work into the modification of the side chains obtained directly from the Knorr synthesis.
In the second strategy developed by the groups of Plieninger[17] and of Evans[18] the pyrrole ring is formed by condensation of a C3-unit with a C-N-unit. In one case the variant of Kleinspehn of the Knorr synthesis is used, whereas in the second case the ring closure is achieved in a stepwise fashion. In this strategy both the acetic acid and the propionic acid side chains are in place right from the beginning.
The third strategy is due to Frydman and Rapoport.[12] They started with a pyridine derivative, which they successfully transformed into a suitably substituted azaindole. Hydrogenation of the azaindole led to the porphobilinogen lactam, which could be hydrolysed to porphobilinogen (4).
The forth strategy stems from Anderson and collaborators, who started with the unsubstituted pyrrole.[19,20] Introducing step by step the acetic acid side chain in the b-position, the nitrile group as precursor of the methylamino group in the a-position and the propionic acid side chain in the b'-position finally gave porphobilinogen (4).
The fifth and the sixth strategies were developed by the groups of Adamckzyk[21] and Ganem[22] and were published in the same year. Both strategies use a formal [2 + 3]-cycloaddition of the anion generated from a substituted methyl isocyanide to construct the pyrrole ring. In one approach the propionic acid side chain has to be built up, after the cycloaddition, whereas in the other approach all carbon atoms are present already at this stage.
Comparing the strategy used in the biosynthesis with the strategies developed during
the last 40 years by chemists allows to draw two important conclusions:
1) The efficiency and beauty of the one step, 100% yield biosynthesis has not been reached
by any of the reported synthesis so far;[23]
2) The sensitivity of porphobilinogen (4) has forced the chemists to introduce stabilising
(protecting) groups onto the pyrrole ring in order to be able to manipulate and to isolate
the advanced intermediates. The second restriction by necessity leads to longer synthetic
pathways and the yield of the necessary deprotection step is often rather low.
Therefore the synthesis of porphobilinogen (4) is a double challenge:
1) Will chemists be able to use the beautiful strategy developed by nature and
2) an even more fundamental question: How did nature find this highly efficient and
convergent synthetic strategy?
![]() Development of a potentially biomimetic methodology
Development of a potentially biomimetic methodology
![]() "A Novel Synthesis of Porphobilinogen: Synthetic And Biosynthetic
Studies"
"A Novel Synthesis of Porphobilinogen: Synthetic And Biosynthetic
Studies"
Christiane Bobillier Neier / August 1999